Intest Res.
2022 Jul;20(3):361-369. 10.5217/ir.2021.00126.
Intestinal ultrasonography and fecal calprotectin for monitoring inflammation of ileal Crohn’s disease: two complementary tests
- Affiliations
-
- 1Department of Digestive Medicine, Doctor Peset University Hospital, Valencia, Spain
- 2Department of Radiology, Doctor Peset University Hospital, Valencia, Spain
- 3Department of Pharmacy, Doctor Peset University Hospital, Valencia, Spain
- KMID: 2531990
- DOI: http://doi.org/10.5217/ir.2021.00126
Abstract
- Background/Aims
Tight control of inflammation and adjustment of treatment if activity persists is the current strategy for the management of Crohn’s disease (CD). The usefulness of fecal calprotectin (FC) in isolated involvement of the small intestine in CD is controversial. To assess the usefulness of FC to determine the inflammatory activity detected by intestinal ultrasonography (IUS) in ileal CD.
Methods
Patients with exclusively ileal involvement CD who underwent IUS and an FC were prospectively included. Simple ultrasound index was used to determine inflammatory activity. The usual statistical tests for comparison of diagnostic techniques were used.
Results
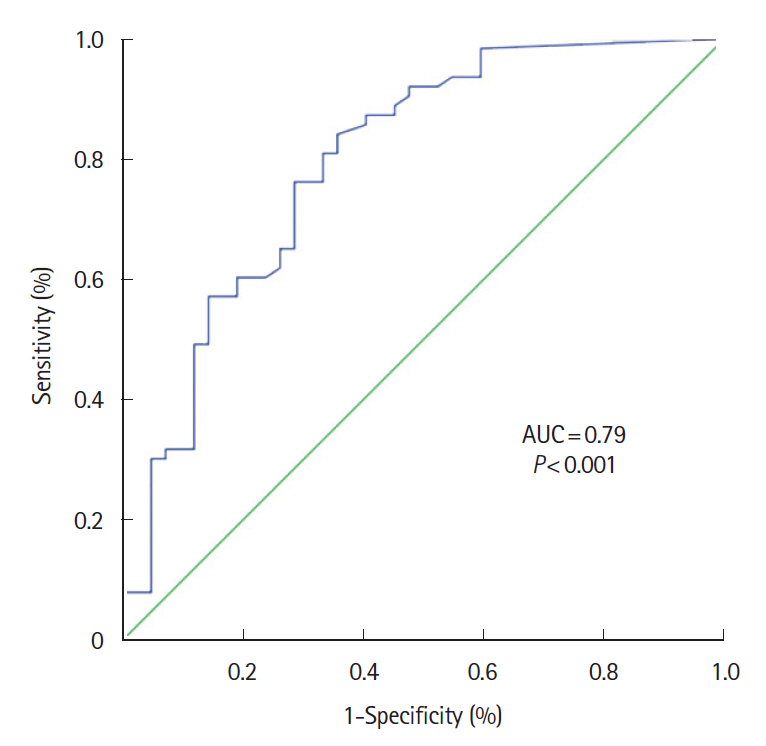
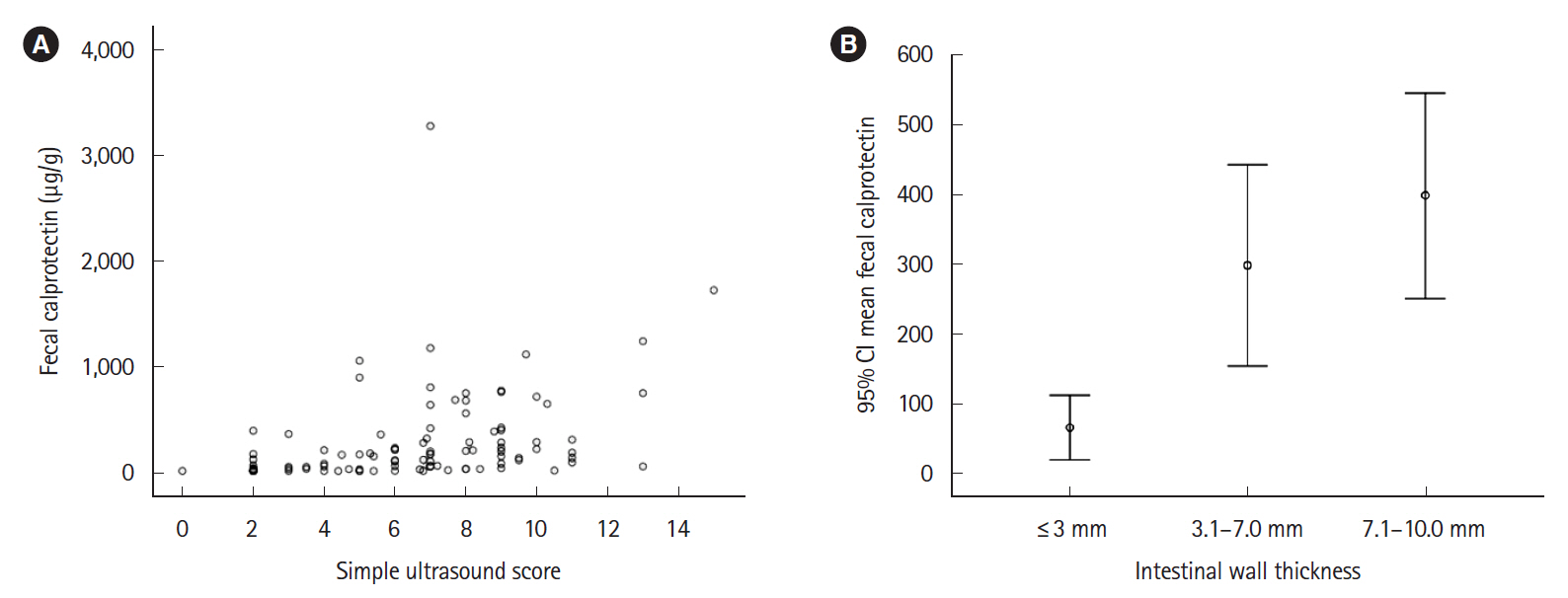
One hundred and five patients were included, IUS showed inflammatory activity in 59% of patients and complications in 18.1%. FC showed a significant correlation with IUS in the weak range (Spearman coefficient r=0.502; P<0.001); the area under the receiver operating characteristic curve was 0.79 (95% confidence interval, 0.70–0.88; P<0.001). The FC value that best reflected the activity in IUS was 100 μg/g with sensitivity, specificity, and positive and negative predictive values of 73.0%, 71.4%, 79.3% and 63.8%, respectively. There were no differences in FC concentration between patients with or without transmural complications. The addition of serum C-reactive protein to FC did not improve the ability to assess IUS activity.
Conclusions
FC has a significant correlation with IUS to monitor ileal CD activity. This correlation is weak and it does not allow assessing the presence of CD complications. Both tests should be used in conjunction for tight control of ileal CD. More studies on noninvasive tests in this location are needed.
Figure
Reference
-
1. Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019; 68:423–433.
Article2. Gonczi L, Bessissow T, Lakatos PL. Disease monitoring strategies in inflammatory bowel diseases: what do we mean by “tight control”? World J Gastroenterol. 2019; 25:6172–6189.
Article3. Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016; 43:317–333.
Article4. Allocca M, Danese S, Laurent V, Peyrin-Biroulet L. Use of cross-sectional imaging for tight monitoring of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020; 18:1309–1323.5. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.
Article6. Bryant RV, Friedman AB, Wright EK, et al. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut. 2018; 67:973–985.
Article7. Ripollés T, Poza J, Suarez Ferrer C, Martínez-Pérez MJ, Martín-Algíbez A, de Las Heras Paez B. Evaluation of Crohn’s disease activity: development of an ultrasound score in a multicenter study. Inflamm Bowel Dis. 2021; 27:145–154.
Article8. Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019; 49:1026–1039.
Article9. Zhulina Y, Cao Y, Amcoff K, Carlson M, Tysk C, Halfvarson J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther. 2016; 44:495–504.
Article10. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017; 390:2779–2789.
Article11. Dulai PS, Singh S, Vande Casteele N, et al. Should we divide Crohn’s disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol. 2019; 17:2634–2643.
Article12. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008; 14:40–46.
Article13. Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015; 50:841–847.
Article14. Simon EG, Wardle R, Thi AA, Eldridge J, Samuel S, Moran GW. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn’s disease? A systematic review. Intest Res. 2019; 17:160–170.
Article15. Makanyanga JC, Pendsé D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014; 24:277–287.
Article16. Cerrillo E, Beltrán B, Pous S, et al. Fecal calprotectin in ileal Crohn’s disease: relationship with magnetic resonance enterography and a pathology score. Inflamm Bowel Dis. 2015; 21:1572–1579.17. Ye L, Cheng W, Chen BQ, et al. Levels of faecal calprotectin and magnetic resonance enterocolonography correlate with severity of small bowel Crohn’s disease: a retrospective cohort study. Sci Rep. 2017; 7:1970.
Article18. Zittan E, Kelly OB, Gralnek IM, Silverberg MS, Hillary Steinhart A. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open. 2018; 2:201–206.
Article19. Jones GR, Fascì-Spurio F, Kennedy NA, et al. Faecal calprotectin and magnetic resonance enterography in ileal Crohn’s disease: correlations between disease activity and long-term follow-up. J Crohns Colitis. 2019; 13:442–450.
Article20. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6.
Article21. Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010; 8:357–363.
Article22. Reenaers C, Bossuyt P, Hindryckx P, Vanpoucke H, Cremer A, Baert F. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United European Gastroenterol J. 2018; 6:1117–1125.
Article23. Nylund K, Maconi G, Hollerweger A, et al. EFSUMB recommendations and guidelines for gastrointestinal ultrasound. Ultraschall Med. 2017; 38:273–284.24. Fraquelli M, Colli A, Casazza G, et al. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005; 236:95–101.
Article25. Patriquin HB, Garcier JM, Lafortune M, et al. Appendicitis in children and young adults: Doppler sonographic-pathologic correlation. AJR Am J Roentgenol. 1996; 166:629–633.
Article26. Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011; 34:125–145.
Article27. Watanabe K. Clinical management for small bowel of Crohn’s disease in the treat-to-target era: now is the time to optimize treatment based on the dominant lesion. Intest Res. 2020; 18:347–354.
Article28. Dillman JR, Dehkordy SF, Smith EA, et al. Defining the ultrasound longitudinal natural history of newly diagnosed pediatric small bowel Crohn disease treated with infliximab and infliximab-azathioprine combination therapy. Pediatr Radiol. 2017; 47:924–934.
Article29. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article30. Mak LY, Tong TS, Cheung KS, et al. Combined use of common fecal and blood markers for detection of endoscopically active inflammatory bowel disease. Clin Transl Gastroenterol. 2020; 11:e00138.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fecal Calprotectin in Inflammatory Bowel Disease
- Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease
- Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis
- Combination of leucine-rich alpha-2 glycoprotein and fecal markers detect Crohn’s disease activity confirmed by balloon-assisted enteroscopy
- Clinical Utility of Fecal Neutrophil Gelatinase-Associated Lipocalin and Calprotectin as Biomarkers of Clostridioides (Clostridium) difficile Infection