Obstet Gynecol Sci.
2022 Jul;65(4):376-381. 10.5468/ogs.22012.
A modified hydrostatic microfluidic pumpless device for in vitro murine ovarian tissue culture as research model for fertility preservation
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 2Research Unit of Reproductive Medicine and Fertility Preservation, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 3Micro/Nano Electromechanical Integrated Device Research Unit, Department of Mechanical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok, Thailand
- 4Department of Anatomy, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand
- KMID: 2531846
- DOI: http://doi.org/10.5468/ogs.22012
Abstract
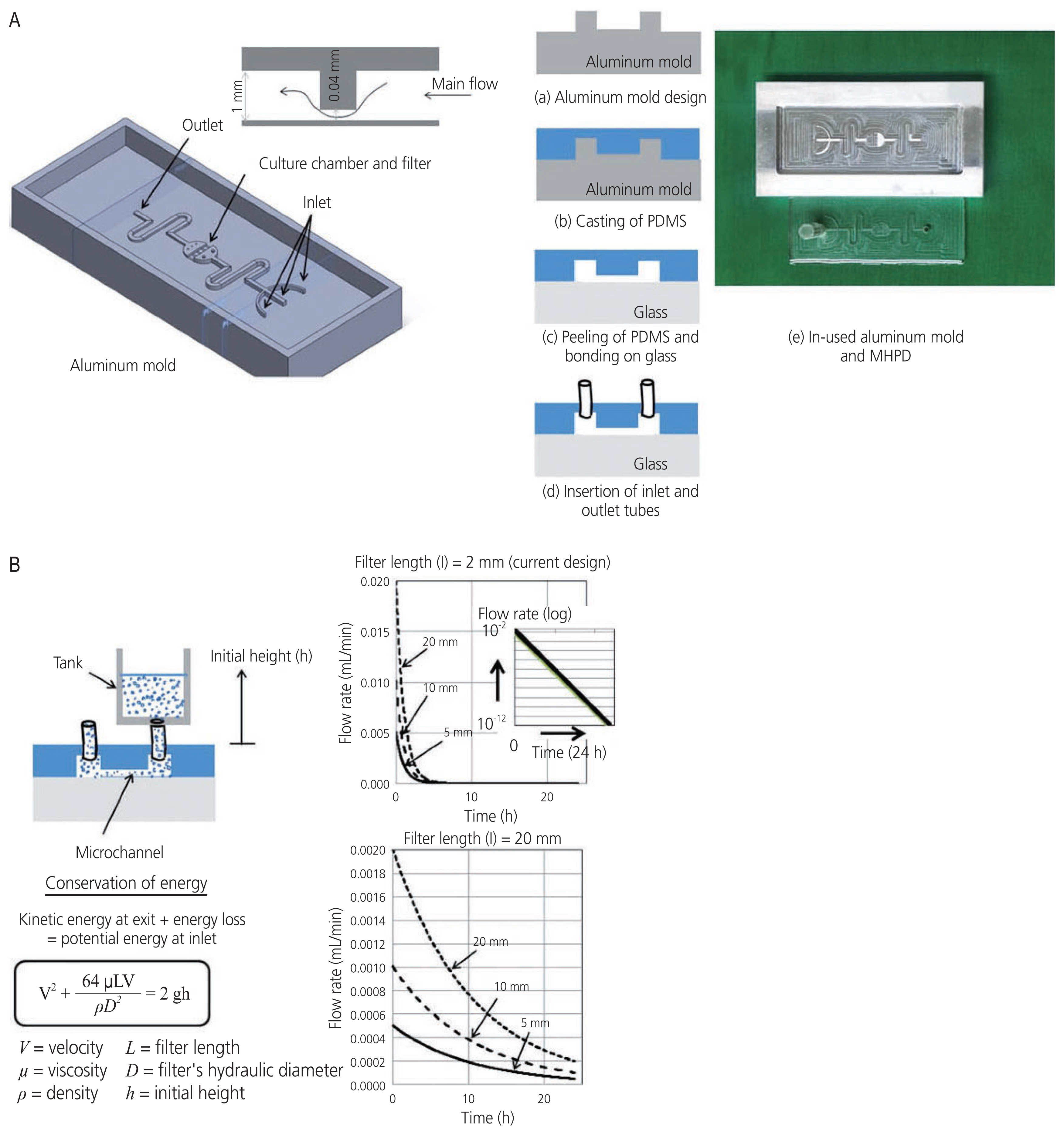
- This study aimed to compare the efficacies of conventional and non-conventional (modified hydrostatic microfluidic pumpless device, MHPD) systems on ovarian tissue culture and in vitro follicle growth using a mouse model. A total of 56 ovarian cortical tissues retrieved from seven wild-type mice were divided into three groups: 1) fresh control, 2) conventional culture system (control), and 3) non-conventional system with MHPD. Ovarian tissues were cultured for 96 hours and evaluated for follicle morphology, developmental stage, intact follicle density, and relative gene expression levels (proliferating cell nuclear antigen, insulin like growth factor 1, BAX, and Bcl-2). Our major data demonstrated that the mean percentage of primary follicle development was increased by the MHPD (P<0.05). In addition, this device could maintain and support follicle development better than the conventional culture systems. However, the overall outcomes were not significantly improved by our first-design prototype. Consequently, nextgeneration platforms should be developed as alternative medical tools for fertility preservation research.
Figure
Reference
-
References
1. Ataman LM, Rodrigues JK, Marinho RM, Caetano JP, Chehin MB, Alves da Motta EL, et al. Creating a global community of practice for oncofertility. J Glob Oncol. 2016; 2:83–96.
Article2. Anderson RA, Wallace WHB, Telfer EE. Ovarian tissue cryopreservation for fertility preservation: clinical and research perspectives. Hum Reprod Open. 2017; 2017:hox001.
Article3. Obermair A, Baxter E, Brennan DJ, McAlpine JN, Muellerer JJ, Amant F, et al. Fertility-sparing treatment in early endometrial cancer: current state and future strategies. Obstet Gynecol Sci. 2020; 63:417–31.
Article4. Telfer EE. Future developments: in vitro growth (IVG) of human ovarian follicles. Acta Obstet Gynecol Scand. 2019; 98:653–8.
Article5. Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010; 16:395–414.
Article6. Filatov MA, Khramova YV, Kiseleva MV, Malinova IV, Komarova EV, Semenova ML. Female fertility preservation strategies: cryopreservation and ovarian tissue in vitro culture, current state of the art and future perspectives. Zygote. 2016; 24:635–53.
Article7. Salama M, Woodruff TK. From bench to bedside: current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet Gynecol Scand. 2019; 98:659–64.
Article8. Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017; 8:14584.
Article9. Komeya M, Hayashi K, Nakamura H, Yamanaka H, Sanjo H, Kojima K, et al. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci Rep. 2017; 7:15459.
Article10. Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014; 507:181–9.
Article11. Aziz AUR, Fu M, Deng J, Geng C, Luo Y, Lin B, et al. A microfluidic device for culturing an encapsulated ovarian follicle. Micromachines (Basel). 2017; 8:335.
Article12. Lo BKM, Sheikh S, Williams SA. In vitro and in vivo mouse follicle development in ovaries and reaggregated ovaries. Reproduction. 2019; 157:135–48.
Article13. Sadeghzadeh Oskouei B, Pashaiasl M, Heidari MH, Salehi M, Veladi H, Ghaderi Pakdel F, et al. Evaluation of mouse oocyte in vitro maturation developmental competency in dynamic culture systems by design and construction of a lab on a chip device and its comparison with conventional culture system. Cell J. 2016; 18:205–13.14. Campos Marín A, Brunelli M, Lacroix D. Flow perfusion rate modulates cell deposition onto scaffold substrate during cell seeding. Biomech Model Mechanobiol. 2018; 17:675–87.
Article15. Komeya M, Yamanaka H, Sanjo H, Yao M, Nakamura H, Kimura H, et al. In vitro spermatogenesis in two-dimensionally spread mouse testis tissues. Reprod Med Biol. 2019; 18:362–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ovarian tissue cryopreservation and transplantation
- Microfluidic Systems for Assisted Reproductive Technologies: Advantages and Potential Applications
- Influence of hydrogel encapsulation during cryopreservation of ovarian tissues and impact of post-thawing in vitro culture systems in a research animal model
- History of fertility preservation
- Controlled ovarian hyperstimulation for fertility preservation in women with breast cancer: Practical issues