J Cerebrovasc Endovasc Neurosurg.
2022 Jun;24(2):121-128. 10.7461/jcen.2021.E2021.08.009.
A study on the proper catheter position in minimally invasive surgery using stereotactic aspiration plus urokinase for intracerebral hemorrhage
- Affiliations
-
- 1Department of Neurosurgery, Presbyterian Medical Center, Jeonju, Korea
- 2Department of Neurosurgery, Chonnam National University Hospital and Medical School, Gwangju, Korea
- KMID: 2531654
- DOI: http://doi.org/10.7461/jcen.2021.E2021.08.009
Abstract
Objective
The surgical method for treating spontaneous intracranial hemorrhage (ICH) is not well established despite ICH’s high prevalence and poor prognosis. Minimally invasive surgery has recently received attention; however, literature on this method is scarce. In particular, the appropriate location of the catheter in the hematoma has not been described. We examined whether the catheter position affects the hematoma reduction in a hematoma >50 cc.
Methods
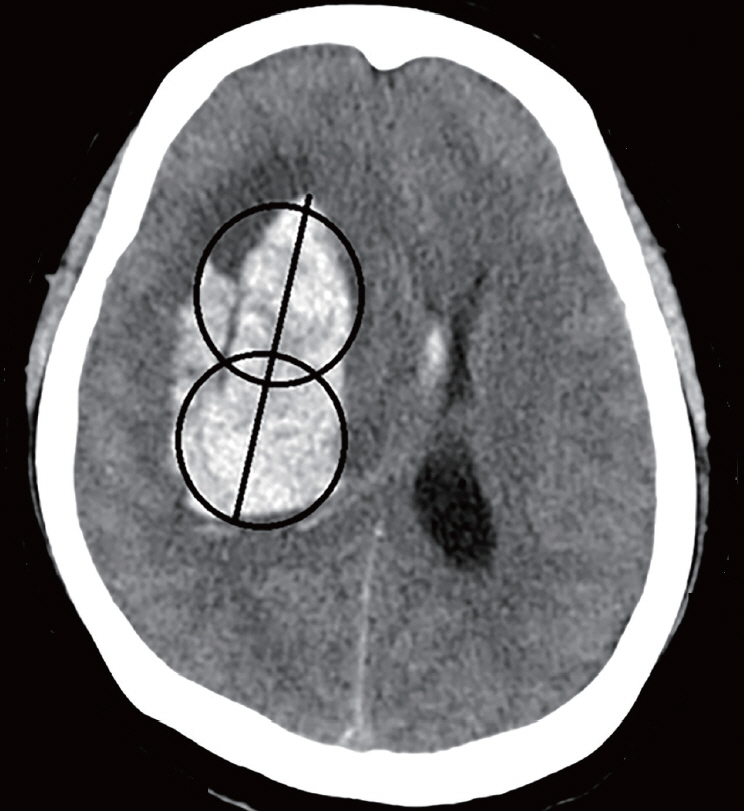
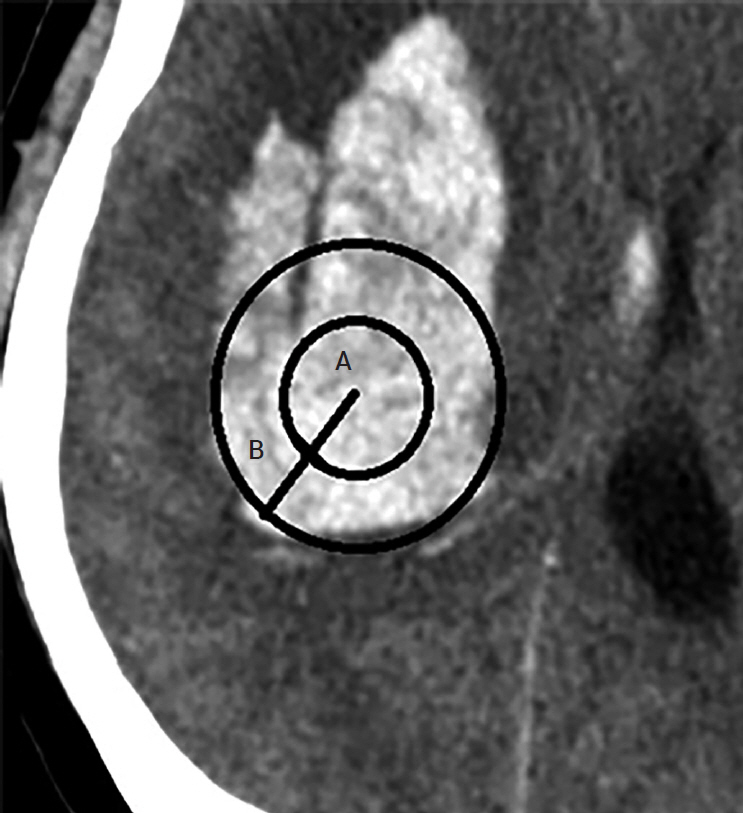
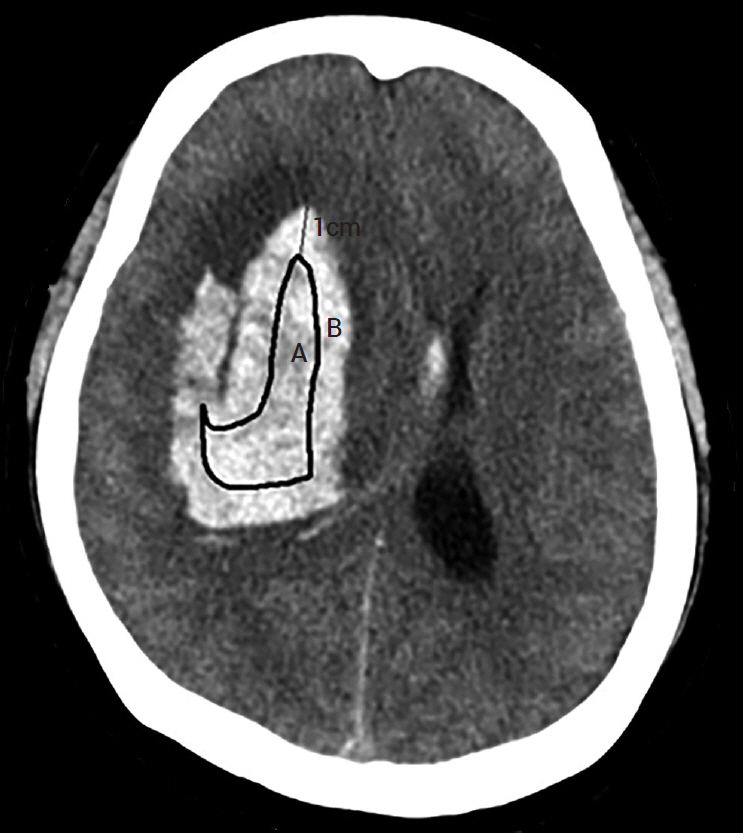
We investigated the prognoses of 36 patients with ICH who underwent stereotactic aspiration and hematoma drainage using urokinase from January 2010 to December 2018 and the hematoma reduction rates according to the tube position. Two methods evaluated the position of the catheter. In the first method, the hematoma was an imaginary sphere. The center point was set as the operation target. We evaluated the catheter position by determining whether it was in the deep part or the outer part of the half point from that location to the hematoma margin. In the second method, we evaluated whether the catheter was located 1 cm inside the hematoma margin.
Results
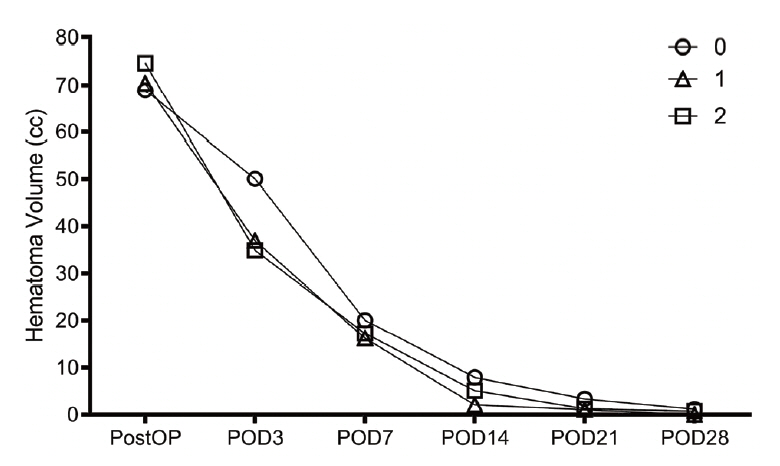
In both the first and second evaluations, there were no differences in age, midline shift, intraventricular hemorrhage status, hematoma volume on admission, Glasgow Coma Scale score on admission, time to operation after symptom onset, and systolic blood pressure. The rates of decrease in bleeding and the prognoses were also not significantly different.
Conclusions
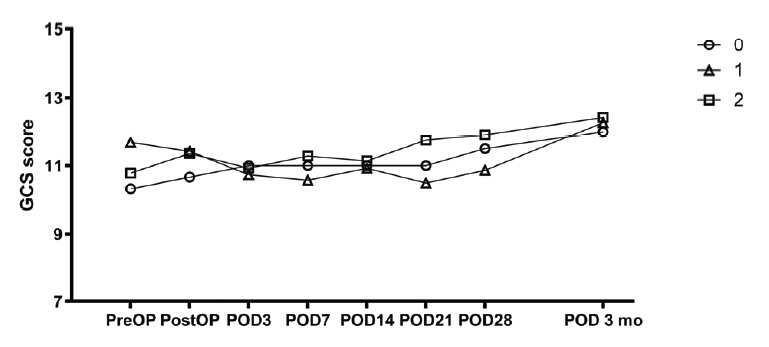
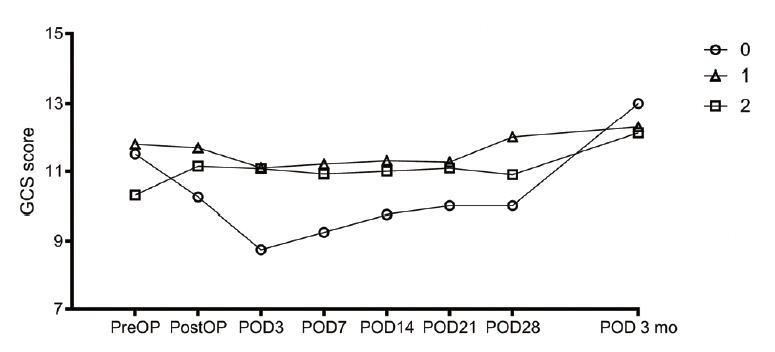
If the catheter is in the hematoma, the rate of hematoma reduction at any position is similar.
Figure
Reference
-
1. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993; Jul. 24(7):987–93.
Article2. Carhuapoma JR, Barrett RJ, Keyl PM, Hanley DF, Johnson RR. Stereotactic aspiration-thrombolysis of intracerebral hemorrhage and its impact on perihematoma brain edema. Neurocrit Care. 2008; 8(3):322–9.
Article3. Fam MD, Hanley D, Stadnik A, Zeineddine HA, Girard R, Jesselson M, et al. Surgical performance in minimally invasive surgery plus recombinant tissue plasminogen activator for intracerebral hemorrhage evacuation phase III clinical trial. Neurosurgery. 2017; Nov. 81(5):860–6.
Article4. Fung C, Murek M, Z’Graggen WJ, Krähenbühl AK, Gautschi OP, Schucht P, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke. 2012; Dec. 43(12):3207–11.
Article5. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019; 393(10175):1021–32.6. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; Jul. 46(7):2032–60.
Article7. Hondo H, Uno M, Sasaki K, Ebisudani D, Shichijo F, Toth Z, et al. Computed tomography controlled aspiration surgery for hypertensive intracerebral hemorrhage. Experience of more than 400 cases. Stereotact Funct Neurosurg. 1990; 54-55:432–7.
Article8. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; Aug. 27(9):1304–5.
Article9. Manno EM. Update on intracerebral hemorrhage. Continuum (Minneap Minn). 2012; Jun. 18(3):598–610.
Article10. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013; Aug. 382(9890):397–408.
Article11. Morgan T, Zuccarello M, Narayan R, Keyl P, Lane K, Hanley D. Preliminary findings of the minimally-invasive surgery plus rtPA for intracerebral hemorrhage evacuation (MISTIE) clinical trial. Acta Neurochir Suppl. 2008; 105:147–51.
Article12. Morgenstern LB, Hemphill JC III, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010; Jul. 41(9):2108–29.
Article13. Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013; Mar. 44(3):627–34.
Article14. Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: a prospective randomized study. Surg Neurol. 2006; Nov. 66(5):492–501. discussion 501.
Article15. Teernstra OP, Evers SMAA, Lodder J, Leffers P, Franke CL, Blaauw G, et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke. 2003; Apr. 34(4):968–74.
Article16. Vespa P, McArthur D, Miller C, O’Phelan K, Frazee J, Kidwell C, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005; 2(3):274–81.
Article17. Wang W-Z, Jiang B, Liu H-M, Li D, Lu C-Z, Zhao Y-D, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke. 2009; Feb. 4(1):11–6.
Article18. Xu H, Li R, Duan Y, Wang J, Liu S, Zhang Y, et al. Quantitative assessment on blood-brain barrier permeability of acute spontaneous intracerebral hemorrhage in basal ganglia: a CT perfusion study. Neuroradiology. 2017; Jul. 59(7):677–84.
Article19. Zhou H, Zhang Y, Liu L, Han X, Tao Y, Tang Y, et al. A prospective controlled study: minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol. 2011; Jun. 11:76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Analysis of Stereotactic aspiration in the Management of Spontaneous Intracerebral Hemorrhage
- Navigation-assisted Aspiration and Thrombolysis of Deep Intracerebral Hemorrhage
- Stereotactic Endoscopic Removal of Hypertensive Intracerebral Hemorrhage
- Comparative Study on Frameless Stereotactic Hematoma Aspiration versus Frame-Based Stereotactic Hematoma Aspiration in Deep Seated Intracerebral Hemorrhage: A Clinical Article
- Streotactic Evacuation and Urokinase Irrigation in the Management of Spontaneous Intracerebral Hemorrhage