J Korean Diabetes.
2022 Jun;23(2):144-152. 10.4093/jkd.2022.23.2.144.
Aceruloplasminemia Presents as Type 2 Diabetes Associated with Unexplained Microcytic Anemia: A Case Report
- Affiliations
-
- 1Department of Radiology, Presbyterian Medical Center, Jeonju, Korea
- 2Department of Internal Medicine, Presbyterian Medical Center, Jeonju, Korea
- KMID: 2531499
- DOI: http://doi.org/10.4093/jkd.2022.23.2.144
Abstract
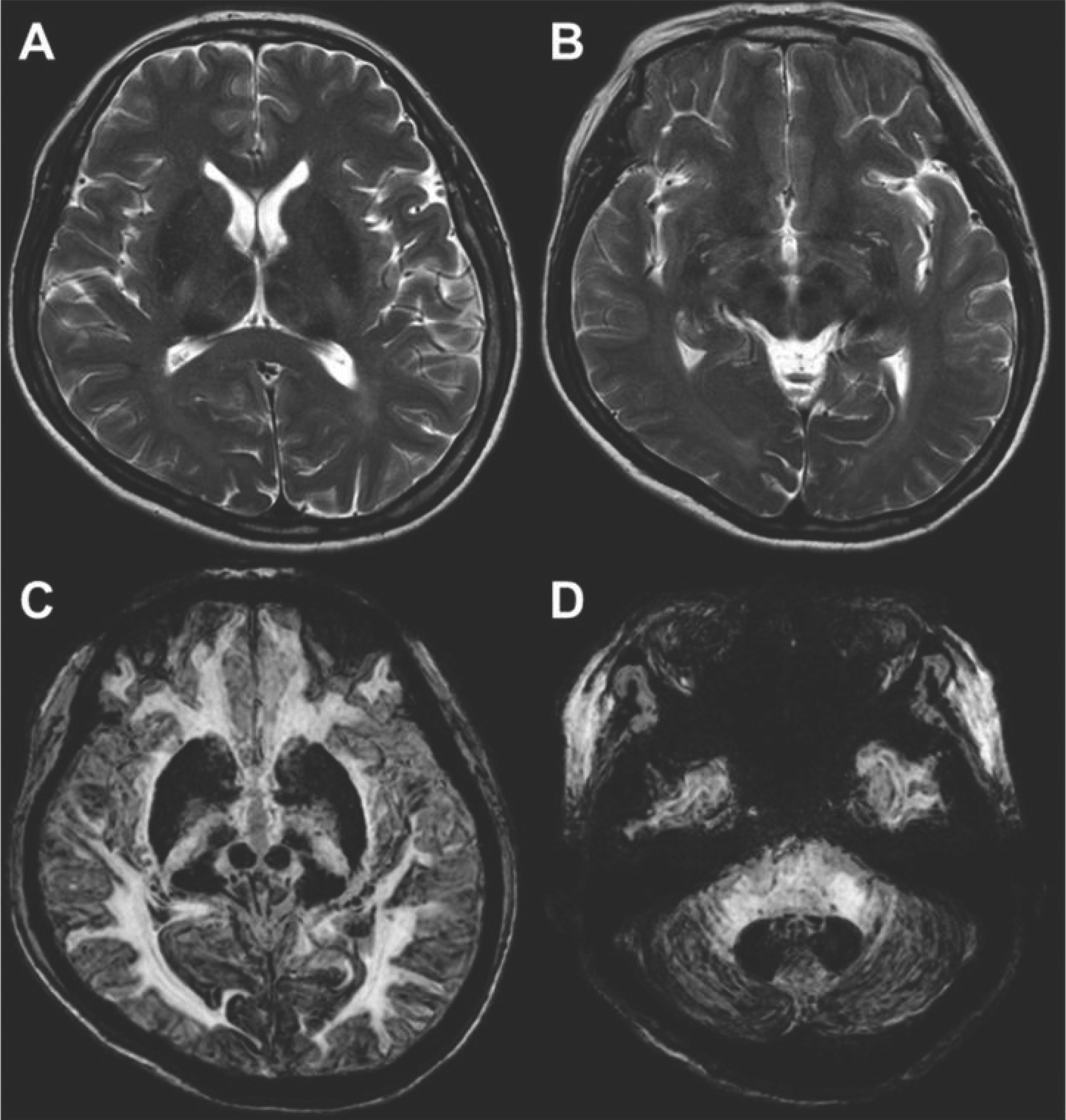
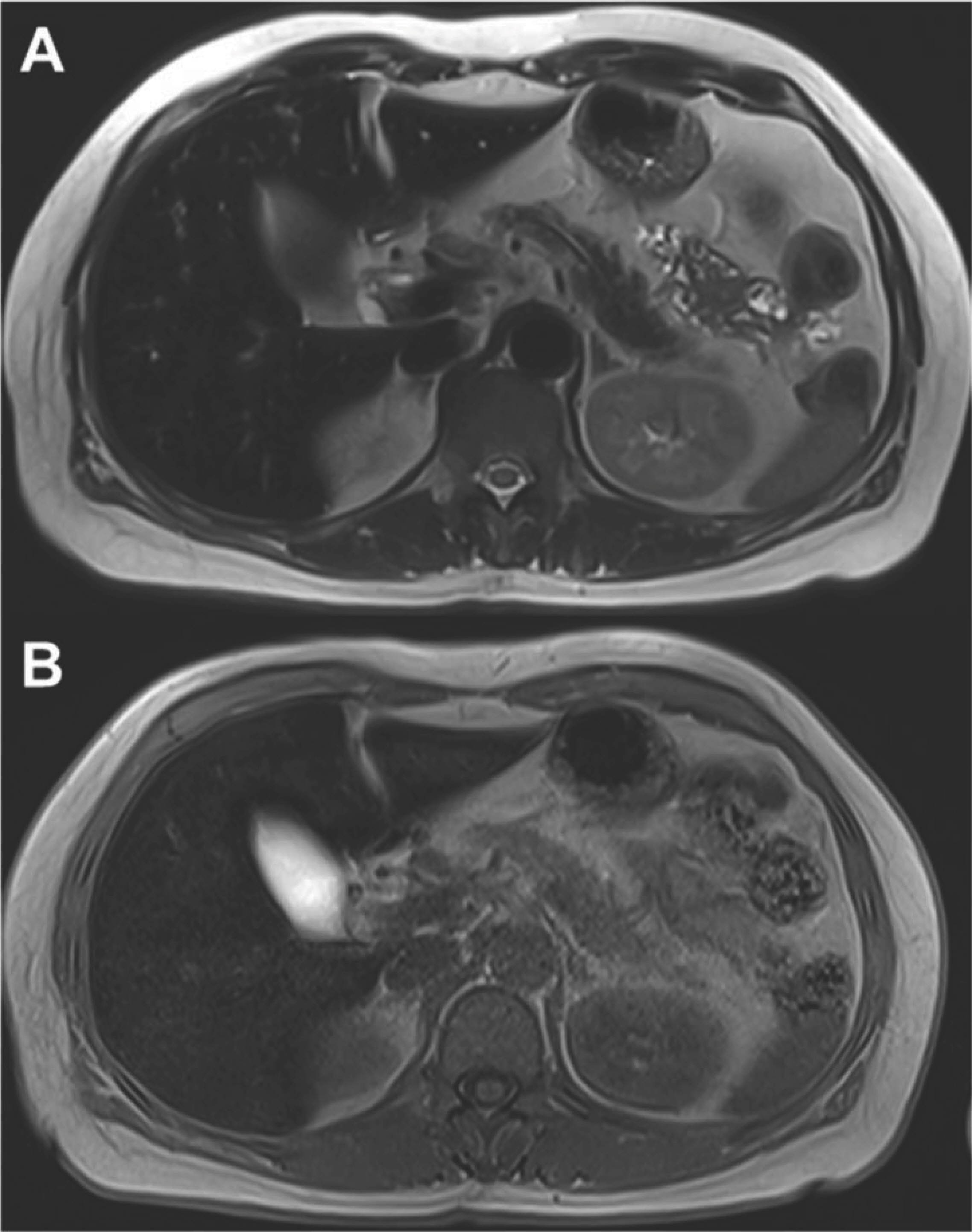
- Aceruloplasminemia (ACP) is a rare genetic disorder characterized by the triad of diabetes mellitus, retinal degeneration, and neurological symptoms. The patient’s clinical and biochemical features highlight substantial phenotype heterogeneity, contributing to the difficulties and delay in diagnosing ACP. We described a patient presenting with diabetes and abnormal iron studies due to ACP with typical neuroradiologic abnormality. A 56-year-old female patient visited our hospital with complaints of weight loss and anxiety. The leading cause of unintentional weight loss was uncontrolled diabetes. She was treated with oral hypoglycemic agents. Initial blood tests revealed unexplained microcytic anemia and high ferritin levels. We performed magnetic resonance imaging (MRI) of the brain to alleviate her excessive concerns about normal memory loss. We suspected that she might have ACP, based on the results of cortical pencil lining sign of the brain MRI and microcytic anemia with decreased ceruloplasmin (CP) and increased ferritin levels. Sequence analysis of the CP gene revealed homozygosity for c.2630 G>A, confirming the clinical diagnosis of ACP. The patient was started on deferasirox with progressive normalization of ferritin. In conclusion, unexplained anemia, often with microcytosis, diabetes, and typical neuroradiologic findings, is the best clue for early diagnosis of ACP.
Keyword
Figure
Reference
-
1.Fasano A., Colosimo C., Miyajima H., Tonali PA., Re TJ., Bentivoglio AR. Aceruloplasminemia: a novel mutation in a family with marked phenotypic variability. Mov Disord. 2008. 23:751–5.
Article2.Miyajima H., Takahashi Y., Kono S. Aceruloplasminemia, an inherited disorder of iron metabolism. Biometals. 2003. 16:205–13.3.Takahashi Y., Miyajima H., Shirabe S., Nagataki S., Suenaga A., Gitlin JD. Characterization of a nonsense mutation in the ceruloplasmin gene resulting in diabetes and neurodegenerative disease. Hum Mol Genet. 1996. 5:81–4.
Article4.Miyajima H., Nishimura Y., Mizoguchi K., Sakamoto M., Shimizu T., Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology. 1987. 37:761–7.
Article5.Miyajima H., Takahashi Y., Kamata T., Shimizu H., Sakai N., Gitlin JD. Use of desferrioxamine in the treatment of aceruloplasminemia. Ann Neurol. 1997. 41:404–7.
Article6.Kim HK., Ki CS., Kim YJ., Lee MS. Radiological findings of two sisters with aceruloplasminemia presenting with cho-rea. Clin Neuroradiol. 2017. 27:385–8.
Article7.Tajima K., Kawanami T., Nagai R., Horiuchi S., Kato T. He-reditary ceruloplasmin deficiency increases advanced gly-cation end products in the brain. Neurology. 1999. 53:619–22.
Article8.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation be-tween antioxidant enzyme gene expression and antioxi-dative defense status of insulin-producing cells. Diabetes. 1997. 46:1733–42.
Article9.Kato T., Daimon M., Kawanami T., Ikezawa Y., Sasaki H., Maeda K. Islet changes in hereditary ceruloplasmin deficiency. Hum Pathol. 1997. 28:499–502.
Article10.McNeill A., Pandolfo M., Kuhn J., Shang H., Miyajima H. The neurological presentation of ceruloplasmin gene mutations. Eur Neurol. 2008. 60:200–5.
Article11.Miyajima H. Aceruloplasminemia. Neuropathology. 2015. 35:83–90.
Article12.Grisoli M., Piperno A., Chiapparini L., Mariani R., Savoiar-do M. MR imaging of cerebral cortical involvement in aceruloplasminemia. AJNR Am J Neuroradiol. 2005. 26:657–61.13.Vila Cuenca M., Marchi G., Barqué A., Esteban-Jurado C., Marchetto A., Giorgetti A. Genetic and clinical heterogeneity in thirteen new cases with aceruloplasminemia. Atypical anemia as a clue for an early diagnosis. Int J Mol Sci. 2020. 21:2374.
Article14.Vroegindeweij LH., van der Beek EH., Boon AJ., Hoogen-doorn M., Kievit JA., Wilson JH. Aceruloplasminemia presents as Type 1 diabetes in non-obese adults: a detailed case series. Diabet Med. 2015. 32:993–1000.
Article15.Muroi R., Yagyu H., Kobayashi H., Nagata M., Sato N., Ideno J. Early onset insulin-dependent diabetes mellitus as an initial manifestation of aceruloplasminaemia. Diabet Med. 2006. 23:1136–9.
Article16.Ogimoto M., Anzai K., Takenoshita H., Kogawa K., Akehi Y., Yoshida R. Criteria for early identification of aceruloplasminemia. Intern Med. 2011. 50:1415–8.
Article17.Pelucchi S., Mariani R., Ravasi G., Pelloni I., Marano M., Tremolizzo L. Phenotypic heterogeneity in seven Italian cases of aceruloplasminemia. Parkinsonism Relat Disord. 2018. 51:36–42.
Article18.Chen M., Zheng J., Liu G., Xu E., Wang J., Fuqua BK. Ceruloplasmin and hephaestin jointly protect the exo-crine pancreas against oxidative damage by facilitating iron efflux. Redox Biol. 2018. 17:432–9.
Article19.Piperno A., Alessio M. Aceruloplasminemia: waiting for an efficient therapy. Front Neurosci. 2018. 12:903.
Article20.Stokes RA., Cheng K., Deters N., Lau SM., Hawthorne WJ., O'Connell PJ. Hypoxia-inducible factor-1α (HIF-1α) potentiates β-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 2013. 22:253–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Infective Endocarditis associtaed with Microcytic Hypochromic Anemia
- Pyridoxine Refractory Sideroblastic Anemia: Diagnosis and Misdiagnosis
- 2 Cases of Beta-thalassemia Minor in Korea
- Modified classification of anemia by RDW
- Clinical significance of RDW in childhood microcytic hypochromic anemia