Korean J Transplant.
2022 Jun;36(2):82-98. 10.4285/kjt.22.0013.
Immune checkpoint inhibitors for solid organ transplant recipients: clinical updates
- Affiliations
-
- 1Transplantation Research Center, Division of Renal Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
- 2Section of GI Oncology, Department of Medical Oncology, Houston Methodist Cancer Center, Houston, TX, USA
- 3Section of Nephrology, Division of Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- 4Division of Kidney Diseases and Hypertension, Donald and Barbara Zucker School of Medicine at Hofstra/ Northwell, Great Neck, NY, USA
- 5Department of Internal Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, NY, USA
- KMID: 2531131
- DOI: http://doi.org/10.4285/kjt.22.0013
Abstract
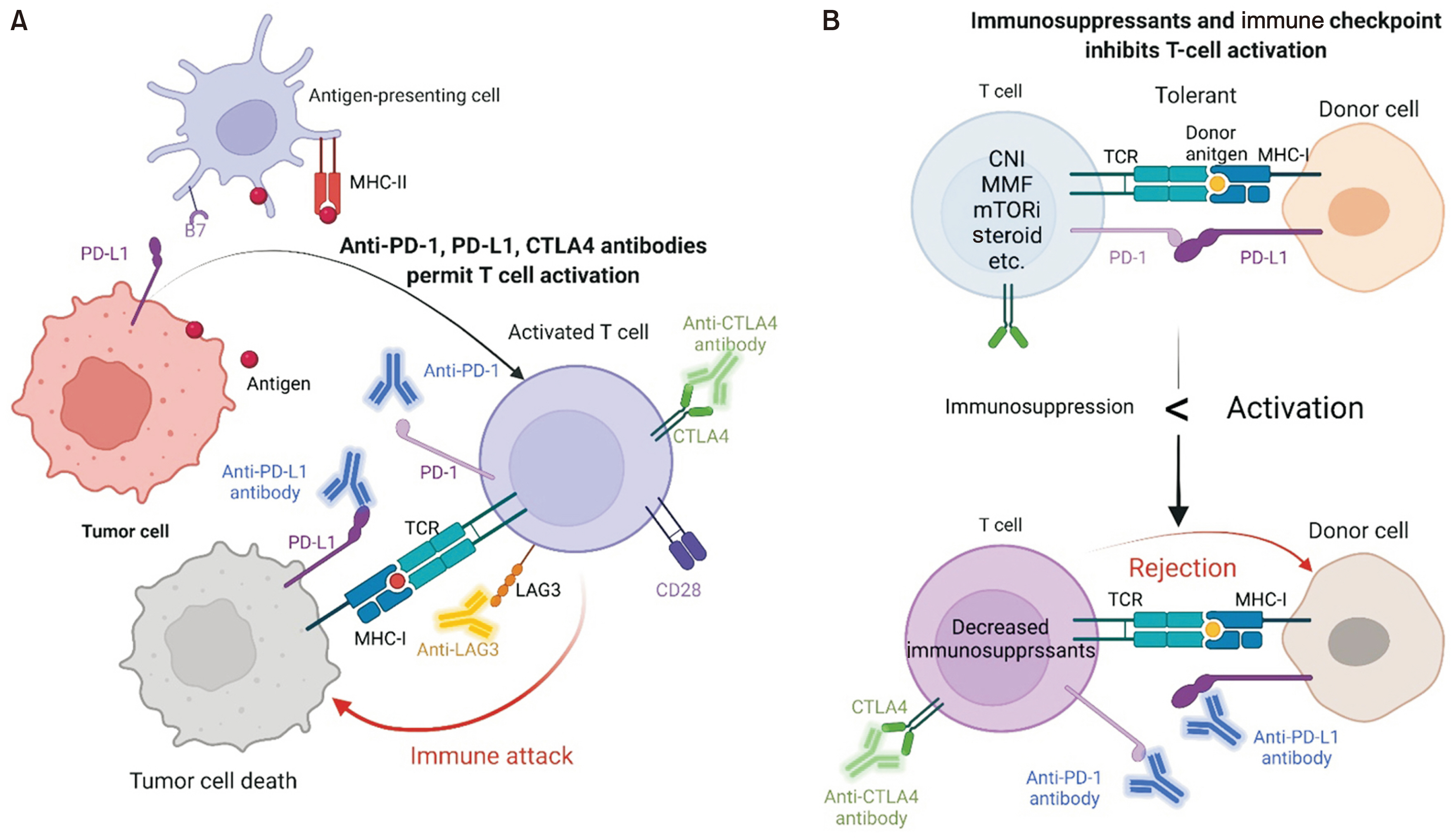
- Transplant care continues to advance with increasing clinical experience and improvements in immunosuppressive therapy. As the population ages and long-term survival improves, transplant patient care has become more complex due to comorbidities, frailty, and the increased prevalence of cancer posttransplantation. Immune checkpoint inhibitors (ICIs) have become a standard treatment option for many cancers in non-transplant patients, but the use of ICIs in transplant patients is challenging due to the possibility of disrupting immune tolerance. However, over the past few years, ICIs have gradually started to be used in transplant patients as well. In this study, we review the current use of ICIs after all solid organ transplantation procedures (kidney, liver, heart, and lung). Increasing data suggest that the type and number of immunosuppressants may affect the risk of rejection after immunotherapy. Immunotherapy for cancer in transplant patients may be a feasible option for selected patients; however, prospective trials in specific organ transplant recipients are needed.
Keyword
Figure
Reference
-
1. Twomey JD, Zhang B. 2021; Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 23:39. DOI: 10.1208/s12248-021-00574-0. PMID: 33677681. PMCID: PMC7937597.2. Haslam A, Prasad V. 2019; Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2:e192535. DOI: 10.1001/jamanetworkopen.2019.2535. PMID: 31050774. PMCID: PMC6503493.3. Murakami N, Motwani S, Riella LV. 2017; Renal complications of immune checkpoint blockade. Curr Probl Cancer. 41:100–10. DOI: 10.1016/j.currproblcancer.2016.12.004. PMID: 28189263. PMCID: PMC5440194.4. Lichtenegger FS, Rothe M, Schnorfeil FM, Deiser K, Krupka C, Augsberger C, et al. 2018; Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front Immunol. 9:385. DOI: 10.3389/fimmu.2018.00385. PMID: 29535740. PMCID: PMC5835137.5. Food and Drug Administration (FDA). Drugs@FDA: FDA-approved drugs [Internet]. Silver Spring, MA: FDA;cited 2022 Jan 25. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. 2021; Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–49. DOI: 10.3322/caac.21660. PMID: 33538338.7. Chapman JR, Webster AC, Wong G. 2013; Cancer in the transplant recipient. Cold Spring Harb Perspect Med. 3:a015677. DOI: 10.1101/cshperspect.a015677. PMID: 23818517. PMCID: PMC3685882.8. Pavlakis M, Michaels MG, Tlusty S, Turgeon N, Vece G, Wolfe C, et al. 2019; Renal cell carcinoma suspected at time of organ donation 2008-2016: a report of the OPTN ad hoc Disease Transmission Advisory Committee Registry. Clin Transplant. 33:e13597. DOI: 10.1111/ctr.13597. PMID: 31104323.9. Eccher A, Girolami I, Motter JD, Marletta S, Gambaro G, Momo RE, et al. 2020; Donor-transmitted cancer in kidney transplant recipients: a systematic review. J Nephrol. 33:1321–32. DOI: 10.1007/s40620-020-00775-4. PMID: 32535833. PMCID: PMC7701067.10. Eccher A, Girolami I, Marletta S, Brunelli M, Carraro A, Montin U, et al. 2021; Donor-transmitted cancers in transplanted livers: analysis of clinical outcomes. Liver Transpl. 27:55–66. DOI: 10.1002/lt.25858. PMID: 32746498.11. Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. 2006; Cancer incidence before and after kidney transplantation. JAMA. 296:2823–31. DOI: 10.1001/jama.296.23.2823. PMID: 17179459.12. Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. 2014; Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 32:e69–71. DOI: 10.1200/JCO.2013.49.2314. PMID: 24493726. PMCID: PMC4870592.13. De Bruyn P, Van Gestel D, Ost P, Kruse V, Brochez L, Van Vlierberghe H, et al. 2019; Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Curr Opin Oncol. 31:54–64. DOI: 10.1097/CCO.0000000000000505. PMID: 30694841.14. Hariharan S, Israni AK, Danovitch G. 2021; Long-term survival after kidney transplantation. N Engl J Med. 385:729–43. DOI: 10.1056/NEJMra2014530. PMID: 34407344.15. Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, et al. 2021; A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. 100:196–205. DOI: 10.1016/j.kint.2020.12.015. PMID: 33359528. PMCID: PMC8222056.16. Lipson EJ, Naqvi FF, Loss MJ, Schollenberger MD, Pardoll DM, Moore J Jr, et al. 2020; Kidney retransplantation after anti-programmed cell death-1 (PD-1)-related allograft rejection. Am J Transplant. 20:2264–8. DOI: 10.1111/ajt.15856. PMID: 32185872. PMCID: PMC7395855.17. Lipson EJ, Bagnasco SM, Moore J Jr, Jang S, Patel MJ, Zachary AA, et al. 2016; Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med. 374:896–8. DOI: 10.1056/NEJMc1509268. PMID: 26962927. PMCID: PMC4850555.18. Nguyen LS, Ortuno S, Lebrun-Vignes B, Johnson DB, Moslehi JJ, Hertig A, et al. 2021; Transplant rejections associated with immune checkpoint inhibitors: a pharmacovigilance study and systematic literature review. Eur J Cancer. 148:36–47. DOI: 10.1016/j.ejca.2021.01.038. PMID: 33721705.19. Saberianfar S, Nguyen LS, Manouchehri A, Lebrun-Vignes B, Moslehi JJ, Johnson DB, et al. 2020; Solid organ transplant rejection associated with immune-checkpoint inhibitors. Ann Oncol. 31:543–4. DOI: 10.1016/j.annonc.2020.01.012. PMID: 32061451.20. Melancon JK, Khalil A, Lerman MJ. 2020; Donor-derived cell free DNA: is it all the same? Kidney360. 1:1118–23. DOI: 10.34067/KID.0003512020. PMID: 35368782. PMCID: PMC8815488.21. Hurkmans DP, Verhoeven JG, de Leur K, Boer K, Joosse A, Baan CC, et al. 2019; Donor-derived cell-free DNA detects kidney transplant rejection during nivolumab treatment. J Immunother Cancer. 7:182. DOI: 10.1186/s40425-019-0653-6. PMID: 31300068. PMCID: PMC6626432.22. Lakhani L, Alasfar S, Bhalla A, Aala A, Rosenberg A, Ostrander D, et al. 2021; Utility of serial donor-derived cell-free DNA measurements for detecting allograft rejection in a kidney transplant recipient after PD-1 checkpoint inhibitor administration. Transplant Direct. 7:e656. DOI: 10.1097/TXD.0000000000001113. PMID: 33490381. PMCID: PMC7817285.23. Tan B, Baxter M, Casasola R. 2021; Acute renal transplant rejection following nivolumab therapy for metastatic melanoma. BMJ Case Rep. 14:e238037. DOI: 10.1136/bcr-2020-238037. PMID: 33558380. PMCID: PMC7872919.24. Tsung I, Worden FP, Fontana RJ. 2021; A pilot study of checkpoint inhibitors in solid organ transplant recipients with metastatic cutaneous squamous cell carcinoma. Oncologist. 26:133–8. DOI: 10.1002/onco.13539. PMID: 32969143. PMCID: PMC7873324.25. Kumar V, Shinagare AB, Rennke HG, Ghai S, Lorch JH, Ott PA, et al. 2020; The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 25:505–14. DOI: 10.1634/theoncologist.2019-0659. PMID: 32043699. PMCID: PMC7288631.26. Trager MH, Coley SM, Dube G, Khan S, Ingham M, Samie FH, et al. 2020; Combination checkpoint blockade for metastatic cutaneous malignancies in kidney transplant recipients. J Immunother Cancer. 8:e000908. DOI: 10.1136/jitc-2020-000908. PMID: 32503950. PMCID: PMC7279669.27. Venkatachalam K, Malone AF, Heady B, Santos RD, Alhamad T. 2020; Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients. Transplantation. 104:1041–7. DOI: 10.1097/TP.0000000000002914. PMID: 31415036.28. Zehou O, Leibler C, Arnault JP, Sayegh J, Montaudié H, Rémy P, et al. 2018; Ipilimumab for the treatment of advanced melanoma in six kidney transplant patients. Am J Transplant. 18:3065–71. DOI: 10.1111/ajt.15071. PMID: 30107088.29. Barnett R, Barta VS, Jhaveri KD. 2017; Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med. 376:191–2. DOI: 10.1056/NEJMc1614298. PMID: 28076715.30. Kittai AS, Oldham H, Cetnar J, Taylor M. 2017; Immune checkpoint inhibitors in organ transplant patients. J Immunother. 40:277–81. DOI: 10.1097/CJI.0000000000000180. PMID: 28719552.31. Kwatra V, Karanth NV, Priyadarshana K, Charakidis M. 2017; Pembrolizumab for metastatic melanoma in a renal allograft recipient with subsequent graft rejection and treatment response failure: a case report. J Med Case Rep. 11:73. DOI: 10.1186/s13256-017-1229-z. PMID: 28315636. PMCID: PMC5357565.32. Alhamad T, Venkatachalam K, Linette GP, Brennan DC. 2016; Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant. 16:1332–3. DOI: 10.1111/ajt.13711. PMID: 26752406.33. Boils CL, Aljadir DN, Cantafio AW. 2016; Use of the PD-1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant. 16:2496–7. DOI: 10.1111/ajt.13786. PMID: 26988410.34. Herz S, Höfer T, Papapanagiotou M, Leyh JC, Meyenburg S, Schadendorf D, et al. 2016; Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur J Cancer. 67:66–72. DOI: 10.1016/j.ejca.2016.07.026. PMID: 27614165.35. Jose A, Yiannoullou P, Bhutani S, Denley H, Morton M, Picton M, et al. 2016; Renal allograft failure after ipilimumab therapy for metastatic melanoma: a case report and review of the literature. Transplant Proc. 48:3137–41. DOI: 10.1016/j.transproceed.2016.07.019. PMID: 27932166.36. Ong M, Ibrahim AM, Bourassa-Blanchette S, Canil C, Fairhead T, Knoll G. 2016; Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer. 4:64. DOI: 10.1186/s40425-016-0171-8. PMID: 27777773. PMCID: PMC5067882.37. Spain L, Higgins R, Gopalakrishnan K, Turajlic S, Gore M, Larkin J. Acute enal allograft rejection after immune checkpoint inhibitor therapy for etastatic melanoma. Ann Oncol. 2016; 27:1135–7. DOI: 10.1093/annonc/mdw130. PMID: 26951628.38. Trotter JF. 2017; Liver transplantation around the world. Curr Opin Organ Transplant. 22:123–7. DOI: 10.1097/MOT.0000000000000392. PMID: 28151809.39. Graziadei I, Zoller H, Fickert P, Schneeberger S, Finkenstedt A, Peck-Radosavljevic M, et al. 2016; Indications for liver transplantation in adults: recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr. 128:679–90. DOI: 10.1007/s00508-016-1046-1. PMID: 27590261. PMCID: PMC5052293.40. Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, et al. 2022; OPTN/SRTR 2020 annual data report: liver. Am J Transplant. 22 Suppl 2:204–309. DOI: 10.1111/ajt.16978. PMID: 35266621.41. Organ Procurement and Transplantation Network (OPTN). Build advanced [Internet]. Carville, LA: OPTN;cited 2022 Feb 1. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced.42. Abdelrahim M, Esmail A, Abudayyeh A, Murakami N, Saharia A, McMillan R, et al. 2021; Transplant oncology: an evolving field in cancer care. Cancers (Basel). 13:4911. DOI: 10.3390/cancers13194911. PMID: 34638395. PMCID: PMC8508383.43. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. 2017; Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 389:2492–502. DOI: 10.1016/S0140-6736(17)31046-2. PMID: 28434648. PMCID: PMC7539326.44. Crocenzi TS, El-Khoueiry AB, Yau TC, Melero I, Sangro B, Kudo M, et al. 2017; Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J Clin Oncol. 35(15 suppl):4013. DOI: 10.1200/JCO.2017.35.15_suppl.4013.45. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. 2018; Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–52. DOI: 10.1016/S1470-2045(18)30351-6. PMID: 29875066.46. Onuma AE, Zhang H, Huang H, Williams TM, Noonan A, Tsung A. 2020; Immune checkpoint inhibitors in hepatocellular cancer: current understanding on mechanisms of resistance and biomarkers of response to treatment. Gene Expr. 20:53–65. DOI: 10.3727/105221620X15880179864121. PMID: 32340652. PMCID: PMC7284108.47. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. 2020; Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 382:1894–905. DOI: 10.1056/NEJMoa1915745. PMID: 32402160.48. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. 2022; Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 40(4 suppl):379. DOI: 10.1200/JCO.2022.40.4_suppl.379.49. Lerut J, Sanchez-Fueyo A. 2006; An appraisal of tolerance in liver transplantation. Am J Transplant. 6:1774–80. DOI: 10.1111/j.1600-6143.2006.01396.x. PMID: 16889539.50. Ono A, Fujimoto A, Yamamoto Y, Akamatsu S, Hiraga N, Imamura M, et al. 2015; Circulating tumor DNA analysis for liver cancers and its usefulness as a liquid biopsy. Cell Mol Gastroenterol Hepatol. 1:516–34. DOI: 10.1016/j.jcmgh.2015.06.009. PMID: 28210698. PMCID: PMC5301414.51. Bai R, Lv Z, Xu D, Cui J. 2020; Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 8:34. DOI: 10.1186/s40364-020-00209-0. PMID: 32864131. PMCID: PMC7450548.52. Khagi Y, Goodman AM, Daniels GA, Patel SP, Sacco AG, Randall JM, et al. 2017; Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor-based immunotherapy. Clin Cancer Res. 23:5729–36. DOI: 10.1158/1078-0432.CCR-17-1439. PMID: 28972084. PMCID: PMC5678984.53. Bratman SV, Yang SY, Iafolla MA, Liu Z, Hansen AR, Bedard PL, et al. 2020; Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 1:873–81. DOI: 10.1038/s43018-020-0096-5. PMID: 35121950.54. Hsu CH, Lu S, Abbas A, Guan Y, Zhu AX, Aleshin A, et al. 2020; Longitudinal and personalized detection of circulating tumor DNA (ctDNA) for monitoring efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 38(15 suppl):3531. DOI: 10.1200/JCO.2020.38.15_suppl.3531.55. Qiu J, Tang W, Du C. 2020; Immune checkpoint inhibitors in patients with recurrent hepatocellular carcinoma after liver transplantation: a case report and literature review. Curr Cancer Drug Targets. 20:720–7. DOI: 10.2174/1568009620666200520084415. PMID: 32433005.56. Zhuang L, Mou HB, Yu LF, Zhu HK, Yang Z, Liao Q, et al. 2020; Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation. Hepatobiliary Pancreat Dis Int. 19:91–3. DOI: 10.1016/j.hbpd.2019.09.011. PMID: 31706859.57. Biondani P, De Martin E, Samuel D. 2018; Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol. 29:286–7. DOI: 10.1093/annonc/mdx548. PMID: 29293878.58. DeLeon TT, Salomao MA, Aqel BA, Sonbol MB, Yokoda RT, Ali AH, et al. 2018; Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 9:1054–62. DOI: 10.21037/jgo.2018.07.05. PMID: 30603124. PMCID: PMC6286929.59. Gassmann D, Weiler S, Mertens JC, Reiner CS, Vrugt B, Nägeli M, et al. 2018; Liver allograft failure after nivolumab treatment-a case report with systematic literature research. Transplant Direct. 4:e376. DOI: 10.1097/TXD.0000000000000814. PMID: 30255136. PMCID: PMC6092180.60. Kuo JC, Lilly LB, Hogg D. 2018; Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. 28:61–4. DOI: 10.1097/CMR.0000000000000410. PMID: 29140833.61. Rammohan A, Reddy MS, Farouk M, Vargese J, Rela M. 2018; Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: the silver bullet? Hepatology. 67:1166–8. DOI: 10.1002/hep.29575. PMID: 29023959.62. De Toni EN, Gerbes AL. 2017; Tapering of immunosuppression and sustained treatment with nivolumab in a liver transplant recipient. Gastroenterology. 152:1631–3. DOI: 10.1053/j.gastro.2017.01.063. PMID: 28384452.63. Friend BD, Venick RS, McDiarmid SV, Zhou X, Naini B, Wang H, et al. 2017; Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 64:e26682. DOI: 10.1002/pbc.26682. PMID: 28643391.64. Schvartsman G, Perez K, Sood G, Katkhuda R, Tawbi H. 2017; Immune checkpoint inhibitor therapy in a liver transplant recipient with melanoma. Ann Intern Med. 167:361–2. DOI: 10.7326/L17-0187. PMID: 28761949.65. Varkaris A, Lewis DW, Nugent FW. 2017; Preserved liver transplant after PD-1 pathway inhibitor for hepatocellular carcinoma. Am J Gastroenterol. 112:1895–6. DOI: 10.1038/ajg.2017.387. PMID: 29215617.66. Morales RE, Shoushtari AN, Walsh MM, Grewal P, Lipson EJ, Carvajal RD. 2015; Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. 3:22. DOI: 10.1186/s40425-015-0066-0. PMID: 26082835. PMCID: PMC4469313.67. Ranganath HA, Panella TJ. 2015; Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. 38:211. DOI: 10.1097/CJI.0000000000000077. PMID: 25962109.68. Chen GH, Wang GB, Huang F, Qin R, Yu XJ, Wu RL, et al. 2021; Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period. Transpl Immunol. 66:101386. DOI: 10.1016/j.trim.2021.101386. PMID: 33744409.69. Dehghan Y, Schnickel GT, Hosseini M, Burgoyne AM, Ajmera VH, Morris GP, et al. 2021; Rescue liver re-transplantation after graft loss due to severe rejection in the setting of pre-transplant nivolumab therapy. Clin J Gastroenterol. 14:1718–24. DOI: 10.1007/s12328-021-01521-4. PMID: 34643885. PMCID: PMC8557174.70. Qiao ZY, Zhang ZJ, Lv ZC, Tong H, Xi ZF, Wu HX, et al. 2021; Neoadjuvant programmed cell death 1 (PD-1) inhibitor treatment in patients with hepatocellular carcinoma before liver transplant: a cohort study and literature review. Front Immunol. 12:653437. DOI: 10.3389/fimmu.2021.653437. PMID: 34349755. PMCID: PMC8326904.71. Tabrizian P, Florman SS, Schwartz ME. 2021; PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. 21:1979–80. DOI: 10.1111/ajt.16448. PMID: 33316117.72. Nordness MF, Hamel S, Godfrey CM, Shi C, Johnson DB, Goff LW, et al. 2020; Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: are checkpoint inhibitors safe for the pretransplant patient? Am J Transplant. 20:879–83. DOI: 10.1111/ajt.15617. PMID: 31550417.73. Schwacha-Eipper B, Minciuna I, Banz V, Dufour JF. 2020; Immunotherapy as a downstaging therapy for liver transplantation. Hepatology. 72:1488–90. DOI: 10.1002/hep.31234. PMID: 32171041.74. Lewis D, Glehn-Ponsirenas R, Gulbahce N, Hooey LJ, Chaffin JM, Miles J, et al. 2022; High levels of donor-derived cell-free DNA in a case of graft-versus-host-disease following liver transplantation. Am J Transplant. 22:973–6. DOI: 10.1111/ajt.16894. PMID: 34825479.75. Levitsky J, Kandpal M, Guo K, Kleiboeker S, Sinha R, Abecassis M. 2022; Donor-derived cell-free DNA levels predict graft injury in liver transplant recipients. Am J Transplant. 22:532–40. DOI: 10.1111/ajt.16835. PMID: 34510731.76. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. 2016; Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 375:1749–55. DOI: 10.1056/NEJMoa1609214. PMID: 27806233. PMCID: PMC5247797.77. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. 2018; Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–64. DOI: 10.1016/j.jacc.2018.02.037. PMID: 29567210. PMCID: PMC6196725.78. Esfahani K, Buhlaiga N, Thébault P, Lapointe R, Johnson NA, Miller WH Jr. 2019; Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 380:2375–6. DOI: 10.1056/NEJMc1903064. PMID: 31189042.79. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. 2019; Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 380:2377–9. DOI: 10.1056/NEJMc1901677. PMID: 31189043.80. Chambers DC, Perch M, Zuckermann A, Cherikh WS, Harhay MO, Hayes D Jr, et al. 2021; The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult lung transplantation report - 2021 focus on recipient characteristics. J Heart Lung Transplant. 40:1060–72. DOI: 10.1016/j.healun.2021.07.021. PMID: 34446355.81. Ivulich S, Westall G, Dooley M, Snell G. 2018; The evolution of lung transplant immunosuppression. Drugs. 78:965–82. DOI: 10.1007/s40265-018-0930-6. PMID: 29915895.82. Daud A, Mehra MR, Siu A, Johnson MR, Perch M, Budev M, et al. 2020; Immune checkpoint inhibitors in heart or lung transplantation: early results from a registry initiative. J Heart Lung Transplant. 39:604–6. DOI: 10.1016/j.healun.2020.02.015. PMID: 32265077.83. Grant MJ, DeVito N, Salama AK. 2018; Checkpoint inhibitor use in two heart transplant patients with metastatic melanoma and review of high-risk populations. Melanoma Manag. 5:MMT10. DOI: 10.2217/mmt-2018-0004. PMID: 30459942. PMCID: PMC6240846.84. Owonikoko TK, Kumar M, Yang S, Kamphorst AO, Pillai RN, Akondy R, et al. 2017; Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother. 66:45–50. DOI: 10.1007/s00262-016-1918-2. PMID: 27771741. PMCID: PMC5588660.85. Gastman BR, Ernstoff MS. 2016; Tolerability of immune checkpoint inhibition cancer therapy in a cardiac transplant patient. Ann Oncol. 27:2304–5. DOI: 10.1093/annonc/mdw293. PMID: 27502714.86. Qin R, Salama AK. 2015; Report of ipilimumab in a heart transplant patient with metastatic melanoma on tacrolimus. Melanoma Manag. 2:311–4. DOI: 10.2217/mmt.15.27. PMID: 30190859. PMCID: PMC6096438.87. Wong G, Chapman JR. 2008; Cancers after renal transplantation. Transplant Rev (Orlando). 22:141–9. DOI: 10.1016/j.trre.2007.12.004. PMID: 18631867.88. Danesh MJ, Mulvaney PM, Murakami N, Riella LV, Silk AW, Hanna GJ, et al. 2020; Impact of corticosteroids on allograft protection in renal transplant patients receiving anti-PD-1 immunotherapy. Cancer Immunol Immunother. 69:1937–41. DOI: 10.1007/s00262-020-02644-2. PMID: 32588077. PMCID: PMC7479641.89. Xie J, Wang X, Proud CG. 2016; mTOR inhibitors in cancer therapy. F1000Res. 5(F1000 Faculty Rev):2078. DOI: 10.12688/f1000research.9207.1. PMID: 27635236. PMCID: PMC5007757.90. Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. 2012; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 367:329–39. DOI: 10.1056/NEJMoa1204166. PMID: 22830463.91. Esfahani K, Al-Aubodah TA, Thebault P, Lapointe R, Hudson M, Johnson NA, et al. 2019; Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun. 10:4712. DOI: 10.1038/s41467-019-12628-1. PMID: 31624262. PMCID: PMC6797722.92. d'Izarny-Gargas T, Durrbach A, Zaidan M. 2020; Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transplant. 20:2457–65. DOI: 10.1111/ajt.15811. PMID: 32027461.93. Australian New Zealand Clinical Trials Registry (ANZCTR). Trial registered on ANZCTR [Internet]. Camperdown: ANZCTR;cited 2022 Feb 9. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372928&showOriginal=true&isReview=true.94. ClinicalTrials.gov. Home [Internet]. Bethesda, MA: ClinicalTrials.gov;cited 2022 Feb 8. Available from: https://clinicaltrials.gov/ct2/home.95. Adam BA, Murakami N, Reid G, Du K, Jasim R, Boils CL, et al. 2021; Gene expression profiling in kidney transplants with immune checkpoint inhibitor-associated adverse events. Clin J Am Soc Nephrol. 16:1376–86. DOI: 10.2215/CJN.00920121. PMID: 34244334. PMCID: PMC8729568.96. M.D. Anderson Cancer Center. Atezolizumab and bevacizumab before surgery for the treatment of resectable liver cancer. In: ClinicalTrials.gov [Internet]. Bethesda, MD: U.S. National Library of Medicine;2022. cited 2022 Mar 13. Available from: https://clinicaltrials.gov/ct2/show/NCT04721132.97. Williams NC, Tong A, Howard K, Chapman JR, Craig JC, Wong G. 2012; Knowledge, beliefs and attitudes of kidney transplant recipients regarding their risk of cancer. Nephrology (Carlton). 17:300–6. DOI: 10.1111/j.1440-1797.2011.01549.x. PMID: 22171765.98. Sautenet B, Tong A, Manera KE, Chapman JR, Warrens AN, Rosenbloom D, et al. 2017; Developing consensus-based priority outcome domains for trials in kidney transplantation: a multinational delphi survey with patients, caregivers, and health professionals. Transplantation. 101:1875–86. DOI: 10.1097/TP.0000000000001776. PMID: 28738403. PMCID: PMC5603314.99. Howell M, Wong G, Rose J, Tong A, Craig JC, Howard K. 2017; Patient preferences for outcomes after kidney transplantation: a best-worst scaling survey. Transplantation. 101:2765–73. DOI: 10.1097/TP.0000000000001793. PMID: 29064956.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of adverse events in cancer treatment with immune checkpoint inhibitors
- Vaccination strategies in patients with solid organ transplant: evidences and future perspectives
- Current status of cancer immunotherapy with immune checkpoint inhibitors
- Treatment of advanced urogenital cancers with immune checkpoint inhibitors
- Red Blood Cell Autoantibodies in Patients Treated with Immune Checkpoint Inhibitors