Blood Res.
2022 Jun;57(2):129-134. 10.5045/br.2022.2021219.
The impact of nucleic acid testing as a blood donor screening method in transfusion-associated hepatitis C among children with bleeding disorders in Indonesia: a single-center experience
- Affiliations
-
- 1Department of Child Health, Faculty of Medicine University of Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta Pusat, Indonesia
- 2Jakarta Blood Center, Indonesian Red Cross Society, Jakarta Pusat, Indonesia
- KMID: 2530877
- DOI: http://doi.org/10.5045/br.2022.2021219
Abstract
- Background
Children with bleeding disorders, such as hemophilia and von Willebrand disease (VWD), have an increased risk of acquiring transfusion-transmitted infections (TTI). Screening methods to exclude blood donations that are at risk of transmitting infection from donors to recipients are critical to preventing disease transmission. Nucleic acid testing (NAT) is the latest blood donor-screening method. This study aimed to determine the incidence of hepatitis C virus (HCV) infection in children with hemophilia and VWD at Dr. Cipto Mangunkusumo Hospital with a history of blood transfusion before and after implementation of a NAT screening method.
Methods
A cohort retrospective study was conducted on children aged 0‒18 years with bleeding disorders and a history of blood transfusion. In our center, all blood transfusions before 2015 were screened using non-NAT methods, while all blood transfusions were screened using NAT starting in 2015. Eligible patient characteristics were collected from medical records. From July to December 2019, blood samples were obtained from eligible patients for anti-HCV examination. HCV RNA examinations were performed on subjects with reactive anti-HCV results, and the relative risk was calculated.
Results
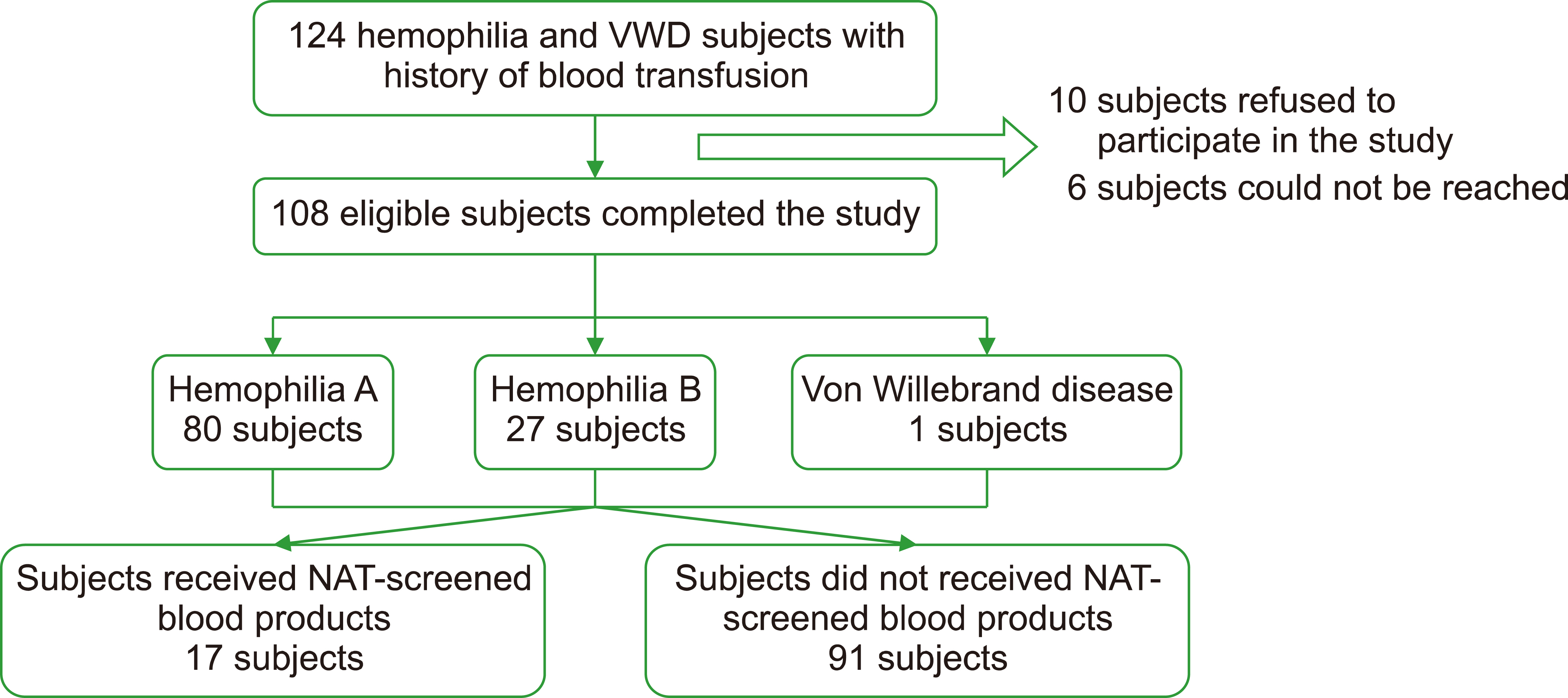
In total, 108 eligible participants were included in this study. We observed that 91 (94.3%) patients had history of receiving non-NAT blood transfusions, while 17 (15.7%) patients received NAT-screened blood transfusions. The proportion of anti-HCV reactivity in the non-NAT group and that in the NAT group were 3.3% (3/91) and 0% (0/17), respectively.
Conclusion
None of the patients exhibited reactivity to anti-HCV after implementing the NAT screening method.
Figure
Cited by 1 articles
-
Transfusion‑transmitted infections
Han Joo Kim, Dae‑Hyun Ko
Blood Res. 2024;59:14. doi: 10.1007/s44313-024-00014-w.
Reference
-
1. Gatot D, Moeslichan MZ. Permono HB, Sutaryo , Ugrasena IDG, Windiastuti E, Abdulsalam M, editors. 2010. Gangguan pembekuan darah yang diturunkan: hemofilia. Buku ajar hematologi onkologi anak. Badan Penerbit Ikatan Dokter Anak Indonesia;Jakarta, Indonesia: p. 174–6.2. Scott JP, Montgomery RR. Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. 2007. Hereditary clotting factor. Nelson textbook of pediatrics. 18th ed. WB Saunders Company;Philadelphia, PA: p. 2066–74. DOI: 10.1016/b978-1-4377-0755-7.00470-x.3. Srivastava A, Santagostino E, Dougall A, et al. 2020; WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 26(Suppl 6):1–158. DOI: 10.1111/hae.14046. PMID: 32744769.
Article4. Lillicrap D, James P. 2009; Von Willebrand disease: an introduction for the primary care physician. Québec, Canada:. World Federation of Hemophilia,. 1–7.5. Farrugia A, Evers T, Falcou PF, Burnouf T, Amorim L, Thomas S. 2009; Plasma fractionation issues. Biologicals. 37:88–93. DOI: 10.1016/j.biologicals.2009.01.005. PMID: 19289290.
Article6. Dean CL, Wade J, Roback JD. 2018; Transfusion-transmitted infections: an update on product screening, diagnostic techniques, and the path ahead. J Clin Microbiol. 56:e00352–18. DOI: 10.1128/JCM.00352-18. PMID: 29669792. PMCID: PMC6018323.
Article7. Toshikuni N, Arisawa T, Tsutsumi M. 2014; Hepatitis C-related liver cirrhosis - strategies for the prevention of hepatic decompensation, hepatocarcinogenesis, and mortality. World J Gastroenterol. 20:2876–87. DOI: 10.3748/wjg.v20.i11.2876. PMID: 24659879. PMCID: PMC3961980.
Article8. Gupta E, Bajpai M, Choudhary A. 2014; Hepatitis C virus: screening, diagnosis, and interpretation of laboratory assays. Asian J Transfus Sci. 8:19–25. DOI: 10.4103/0973-6247.126683. PMID: 24678168. PMCID: PMC3943138.
Article9. Muciño-Bermejo J, Carrillo-Esper R, Uribe M, Méndez-Sánchez N. 2013; Coagulation abnormalities in the cirrhotic patient. Ann Hepatol. 12:713–24. DOI: 10.1016/S1665-2681(19)31312-2. PMID: 24018489.
Article10. Kessler CM. 2006; Update on liver disease in hemophilia patients. Semin Hematol. 43(1 Suppl 1):S13–7. DOI: 10.1053/j.seminhematol.2005.11.021. PMID: 16427378.
Article11. Unit Transfusi Darah Pusat. 2018. Sejarah unit transfusi darah palang merah Indonesia. Unit Transfusi Darah Pusat;Jakarta, Indonesia: at http://home.utdp-pmi.or.id/page/detail/sejarah-utd-pmi. Accessed December 10, 2021.12. Fagan EA. 1991; Testing for hepatitis C virus. BMJ. 303:535–6. DOI: 10.1136/bmj.303.6802.535. PMID: 1655135. PMCID: PMC1670862.
Article13. Kotwal GJ, Baroudy BM, Kuramoto IK, et al. 1992; Detection of acute hepatitis C virus infection by ELISA using a synthetic peptide comprising a structural epitope. Proc Natl Acad Sci U S A. 89:4486–9. DOI: 10.1073/pnas.89.10.4486. PMID: 1374903. PMCID: PMC49107.
Article14. Dufour DR, Talastas M, Fernandez MD, Harris B. 2003; Chemiluminescence assay improves specificity of hepatitis C antibody detection. Clin Chem. 49:940–4. DOI: 10.1373/49.6.940. PMID: 12765991.
Article15. Roth WK. 2019; History and future of nucleic acid amplification technology blood donor testing. Transfus Med Hemother. 46:67–75. DOI: 10.1159/000496749. PMID: 31191192. PMCID: PMC6514489.
Article16. Oswari H, Damardjati F, Gatot D, Munasir Z, Bisanto J. 2007; Clinical and laboratory profiles of hepatitis C in hemophiliac children. Paediatr Indones. 47:229–3. DOI: 10.14238/pi47.5.2007.229-33.
Article17. Senzolo M, Burra P, Cholongitas E, Burroughs AK. 2006; New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol. 12:7725–36. DOI: 10.3748/wjg.v12.i48.7725. PMID: 17203512. PMCID: PMC4087534.
Article18. Dustin LB, Bartolini B, Capobianchi MR, Pistello M. 2016; Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 22:826–32. DOI: 10.1016/j.cmi.2016.08.025. PMID: 27592089. PMCID: PMC5627509.
Article19. Morozov VA, Lagaye S. 2018; Hepatitis C virus: morphogenesis, infection and therapy. World J Hepatol. 10:186–212. DOI: 10.4254/wjh.v10.i2.186. PMID: 29527256. PMCID: PMC5838439.
Article20. Ballani N, Gupta E. 2015; Low signal-to-cutoff ratio (S/Co) in the diagnosis of hepatitis C: a diagnostic dilemma? Indian J Gastroenterol. 34:413–4. DOI: 10.1007/s12664-015-0593-0. PMID: 26498021.
Article21. Choi MS, Lee K, Hong YJ, Song EY, Kim DS, Song J. 2018; The role of the signal-to-cutoff ratio in automated anti-HCV chemiluminescent -immunoassays by referring to the nucleic acid amplification test and the recombinant immunoblot assay. Ann Lab Med. 38:466–72. DOI: 10.3343/alm.2018.38.5.466. PMID: 29797818. PMCID: PMC5973922.
Article22. Zhang M, Rosenberg PS, Brown DL, et al. 2006; Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 107:892–7. DOI: 10.1182/blood-2005-07-2781. PMID: 16204310. PMCID: PMC1895891.
Article23. Yeung LT, To T, King SM, Roberts EA. 2007; Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat. 14:797–805. DOI: 10.1111/j.1365-2893.2007.00873.x. PMID: 17927616.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Qualitative and Quantitative Measurements of Anti-HCV Positive Blood Donor Group
- Analysis of Results of Donor Blood Screening Tests of Hanmaeum Blood Center (2011~2020)
- Performance of HCV and HIV-1 Nucleic Acid Amplification Test(NAT) in Korean Blood Donors
- Necessity of Anti-HBc and Anti-HBs Screening in Korean Blood Donation Program: Study using LG Anti-HBc and LG Anti-HBs ELISA Kit and HBV Nucleic Acid Amplification Test
- Residual Risk of Transfusion-Transmitted Infection with Hepatitis C Virus since the Introduction of Nucleic Acid Testing in Korea