Diabetes Metab J.
2022 May;46(3):391-401. 10.4093/dmj.2022.0048.
Lifestyle Interventions for Non-Obese Patients Both with, and at Risk, of Non-Alcoholic Fatty Liver Disease
- Affiliations
-
- 1NAFLD Research Center, Department of Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Nephrology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 3Section of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Verona, Verona, Italy
- 4Southampton National Institute for Health Research Biomedical Research Centre, University Hospital Southampton, Southampton General Hospital, Southampton, UK
- 5Nutrition and Metabolism, Faculty of Medicine, University of Southampton, Southampton, UK
- 6Institute of Hepatology, Wenzhou Medical University, Wenzhou, China
- 7Key Laboratory of Diagnosis and Treatment for the Development of Chronic Liver Disease in Zhejiang Province, Wenzhou, China
- KMID: 2530178
- DOI: http://doi.org/10.4093/dmj.2022.0048
Abstract
- Non-alcoholic fatty liver disease occurring in non-obese subjects (the so-called non-obese NAFLD) is a highly prevalent but neglected liver condition, which is closely associated with metabolic disorders and suboptimal lifestyles. Landmark studies have shown that lifestyle interventions are potentially beneficial in decreasing the risk of developing non-obese NAFLD and in ameliorating NAFLD in non-obese individuals with pre-existing NAFLD. Lifestyle interventions usually refer to changes in eating habits and physical activity, both of which have a powerful effect on non-obese NAFLD and on risk factors for non-obese NAFLD. However, to date, patients and health-care professionals have a poor awareness and understanding of non-obese NAFLD and the beneficial effects of lifestyle interventions in this patient population. The aim of this narrative review is to briefly discuss the evidence for the effects of lifestyle changes and what changes are needed amongst medical personnel and other stakeholders in order to raise awareness of non-obese NAFLD.
Keyword
Figure
Cited by 1 articles
-
Performance of Simple Fibrosis Score in Non-Alcoholic Fatty Liver Disease with and without Type 2 Diabetes
Seung Min Chung, Min Kyu Kang, Jun Sung Moon, Jung Gil Park
Endocrinol Metab. 2023;38(2):277-281. doi: 10.3803/EnM.2022.1635.
Reference
-
1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018; 15:11–20.
Article2. Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017; 15:474–85.
Article3. Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999-2016. J Intern Med. 2020; 288:139–51.
Article4. Kamada Y, Takahashi H, Shimizu M, Kawaguchi T, Sumida Y, Fujii H, et al. Clinical practice advice on lifestyle modification in the management of nonalcoholic fatty liver disease in Japan: an expert review. J Gastroenterol. 2021; 56:1045–61.
Article5. Zhang X, Goh GB, Chan WK, Wong GL, Fan JG, Seto WK, et al. Unhealthy lifestyle habits and physical inactivity among Asian patients with non-alcoholic fatty liver disease. Liver Int. 2020; 40:2719–31.
Article6. Jung Y, Lee MK, Puri P, Koo BK, Joo SK, Jang SY, et al. Circulating lipidomic alterations in obese and non-obese subjects with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020; 52:1603–14.
Article7. Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020; 11:4982.
Article8. Lin H, Wong GL, Whatling C, Chan AW, Leung HH, Tse CH, et al. Association of genetic variations with NAFLD in lean individuals. Liver Int. 2022; 42:149–60.
Article9. Jung Y, Koo BK, Jang SY, Kim D, Lee H, Lee DH, et al. Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus. Liver Int. 2021; 41:2892–902.
Article10. Chen SD, Zhang H, Rios RS, Li YY, Zhu PW, Jin Y, et al. J-shaped relationship between serum zinc levels and the severity of hepatic necro-inflammation in patients with MAFLD. Nutr Metab Cardiovasc Dis. 2022; 32:1259–65.
Article11. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020; 5:739–52.
Article12. Rios RS, Zheng KI, Zheng MH. Non-alcoholic steatohepatitis and risk of hepatocellular carcinoma. Chin Med J (Engl). 2021; 134:2911–21.
Article13. Wang TY, Wang RF, Bu ZY, Targher G, Byrne CD, Sun DQ, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. 2022; 18:259–68.
Article14. Zheng KI, Zheng MH. The uprising of metabolic dysfunction-associated fatty liver disease (MAFLD) in acute-on-chronic liver failure (ACLF). Hepatobiliary Surg Nutr. 2021; 10:857–9.
Article15. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016; 64:1577–86.
Article16. Ahadi M, Molooghi K, Masoudifar N, Namdar AB, Vossoughinia H, Farzanehfar M. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J Gastroenterol Hepatol. 2021; 36:1497–507.
Article17. Zou TT, Zhang C, Zhou YF, Han YJ, Xiong JJ, Wu XX, et al. Lifestyle interventions for patients with nonalcoholic fatty liver disease: a network meta-analysis. Eur J Gastroenterol Hepatol. 2018; 30:747–55.
Article18. Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018; 69:1349–56.
Article19. Feldman A, Eder SK, Felder TK, Kedenko L, Paulweber B, Stadlmayr A, et al. Clinical and metabolic characterization of lean Caucasian subjects with non-alcoholic fatty liver. Am J Gastroenterol. 2017; 112:102–10.
Article20. Chen F, Esmaili S, Rogers GB, Bugianesi E, Petta S, Marchesini G, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020; 71:1213–27.
Article21. Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: time for reappraisal of BMI-driven approach? Gut. 2022; 71:382–90.
Article22. Tan EX, Lee JW, Jumat NH, Chan WK, Treeprasertsuk S, Goh GB, et al. Non-obese non-alcoholic fatty liver disease (NAFLD) in Asia: an international registry study. Metabolism. 2022; 126:154911.
Article23. Lu FB, Zheng KI, Rios RS, Targher G, Byrne CD, Zheng MH. Global epidemiology of lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2020; 35:2041–50.
Article24. Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M, et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int. 2021; 15:366–79.
Article25. Kuchay MS, Martinez-Montoro JI, Choudhary NS, Fernandez-Garcia JC, Ramos-Molina B. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicines. 2021; 9:1346.
Article26. Lazarus JV, Mark HE, Villota-Rivas M, Palayew A, Carrieri P, Colombo M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol. 2022; 76:771–80.27. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67:328–57.
Article28. Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018; 68:1063–75.
Article29. Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004; 27:205–10.
Article30. Anderson TA. Recent trends in carbohydrate consumption. Annu Rev Nutr. 1982; 2:113–32.
Article31. Yracheta JM, Alfonso J, Lanaspa MA, Roncal-Jimenez C, Johnson SB, Sanchez-Lozada LG, et al. Hispanic Americans living in the United States and their risk for obesity, diabetes and kidney disease: genetic and environmental considerations. Postgrad Med. 2015; 127:503–10.
Article32. Yracheta JM, Lanaspa MA, Le MT, Abdelmalak MF, Alfonso J, Sanchez-Lozada LG, et al. Diabetes and kidney disease in American Indians: potential role of sugar-sweetened beverages. Mayo Clin Proc. 2015; 90:813–23.
Article33. Herman MA, Birnbaum MJ. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021; 33:2329–54.
Article34. Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017; 66:1031–6.
Article35. Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010; 51:1961–71.
Article36. Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020; 130:1453–60.
Article37. Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc. 2016; 91:452–68.38. Kim MS, Krawczyk SA, Doridot L, Fowler AJ, Wang JX, Trauger SA, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest. 2016; 126:4372–86.
Article39. Katz LS, Baumel-Alterzon S, Scott DK, Herman MA. Adaptive and maladaptive roles for ChREBP in the liver and pancreatic islets. J Biol Chem. 2021; 296:100623.
Article40. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010; 7:251–64.
Article41. Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017; 153:743–52.
Article42. Togo J, Hu S, Li M, Niu C, Speakman JR. Impact of dietary sucrose on adiposity and glucose homeostasis in C57BL/6J mice depends on mode of ingestion: liquid or solid. Mol Metab. 2019; 27:22–32.
Article43. Jang C, Wada S, Yang S, Gosis B, Zeng X, Zhang Z, et al. The small intestine shields the liver from fructose-induced steatosis. Nat Metab. 2020; 2:586–93.
Article44. Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J Hepatol. 2019; 71:200–11.
Article45. Guan D, Xiong Y, Borck PC, Jang C, Doulias PT, Papazyan R, et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell. 2018; 174:831–42.
Article46. Guan D, Xiong Y, Trinh TM, Xiao Y, Hu W, Jiang C, et al. The hepatocyte clock and feeding control chronophysiology of multiple liver cell types. Science. 2020; 369:1388–94.
Article47. Reinke H, Asher G. Circadian clock control of liver metabolic functions. Gastroenterology. 2016; 150:574–80.
Article48. Guan D, Lazar MA. Interconnections between circadian clocks and metabolism. J Clin Invest. 2021; 131:e148278.
Article49. Saran AR, Dave S, Zarrinpar A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease. Gastroenterology. 2020; 158:1948–66.
Article50. Bishehsari F, Voigt RM, Keshavarzian A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol. 2020; 16:731–9.
Article51. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016; 23:1048–59.
Article52. Zeb F, Wu X, Chen L, Fatima S, Haq IU, Chen A, et al. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. 2020; 123:1216–26.
Article53. Xin FZ, Zhao ZH, Liu XL, Pan Q, Wang ZX, Zeng L, et al. Escherichia fergusonii promotes nonobese nonalcoholic fatty liver disease by interfering with host hepatic lipid metabolism through its own msRNA 23487. Cell Mol Gastroenterol Hepatol. 2022; 13:827–41.
Article54. Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017; 17:200–7.55. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015; 22:789–98.
Article56. Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007; 56:1729–34.
Article57. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016; 14:290.
Article58. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007; 85:981–8.
Article59. Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018; 6:e1077–86.
Article60. Guthold R, Stevens GA, Riley LM, Bull FC. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health. 2020; 4:23–35.
Article61. World Health Organization Guidelines Review Committee. Global recommendations on physical activity for health. Geneva: World Health Organization;2010.62. Keating SE, Adams LA. Exercise in NAFLD: just do it. J Hepatol. 2016; 65:671–3.
Article63. Berzigotti A, Saran U, Dufour JF. Physical activity and liver diseases. Hepatology. 2016; 63:1026–40.
Article64. Cigrovski Berkovic M, Bilic-Curcic I, Mrzljak A, Cigrovski V. NAFLD and physical exercise: ready, steady, go! Front Nutr. 2021; 8:734859.
Article65. Pan X, Han Y, Zou T, Zhu G, Xu K, Zheng J, et al. Sarcopenia contributes to the progression of nonalcoholic fatty liver disease-related fibrosis: a meta-analysis. Dig Dis. 2018; 36:427–36.
Article66. Li G, Rios RS, Wang XX, Yu Y, Zheng KI, Huang OY, et al. Sex influences the association between appendicular skeletal muscle mass to visceral fat area ratio and non-alcoholic steatohepatitis in patients with biopsy-proven non-alcoholic fatty liver disease. Br J Nutr. 2021; Jun. 28. [Epub]. https://doi.org/10.1017/S0007114521002415.
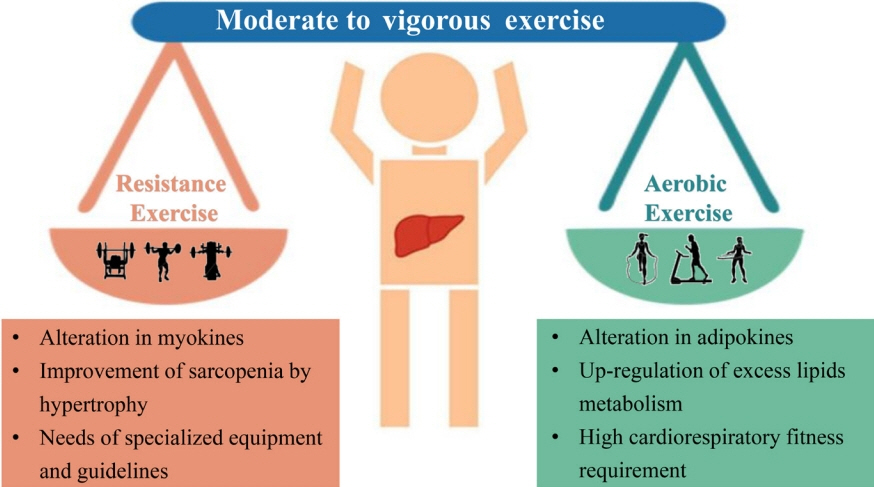
Article67. Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017; 66:142–52.
Article68. Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011; 60:1278–83.
Article69. Lazarus JV, Cortez-Pinto H, Anstee QM. Reply to: “Caveats for the implementation of global strategies against non-alcoholic fatty liver disease”. J Hepatol. 2020; 73:221–2.
Article70. Lazarus JV, Anstee QM, Hagstrom H, Cusi K, Cortez-Pinto H, Mark HE, et al. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol. 2021; 18:717–29.
Article71. Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J (Engl). 2020; 133:2271–73.
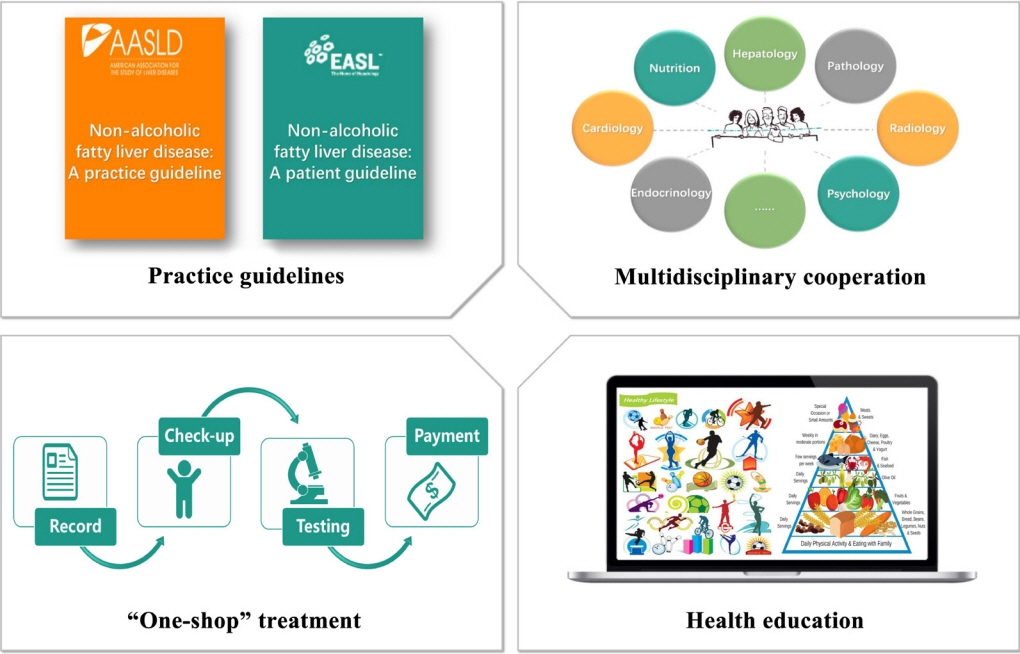
Article72. Lazarus JV, Palayew A, Carrieri P, Ekstedt M, Marchesini G, Novak K, et al. European ‘NAFLD Preparedness Index’: is Europe ready to meet the challenge of fatty liver disease? JHEP Rep. 2021; 3:100234.73. Lazarus JV, Colombo M, Cortez-Pinto H, Huang TT, Miller V, Ninburg M, et al. NAFLD: sounding the alarm on a silent epidemic. Nat Rev Gastroenterol Hepatol. 2020; 17:377–9.74. Kumar S, Wong R, Newberry C, Yeung M, Pena JM, Sharaiha RZ. Multidisciplinary clinic models: a paradigm of care for management of NAFLD. Hepatology. 2021; 74:3472–8.
Article75. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020; 73:202–9.
Article76. Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022; 19:60–78.77. Younossi ZM, Ong JP, Takahashi H, Yilmaz Y, Eguc Hi Y, El Kassas M, et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022; 20:e1456–68.
Article78. Zheng KI, Eslam M, George J, Zheng MH. When a new definition overhauls perceptions of MAFLD related cirrhosis care. Hepatobiliary Surg Nutr. 2020; 9:801–4.
Article79. Moolla A, Motohashi K, Marjot T, Shard A, Ainsworth M, Gray A, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Frontline Gastroenterol. 2019; 10:337–46.
Article80. Armstrong MJ, Hazlehurst JM, Parker R, Koushiappi E, Mann J, Khan S, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014; 107:33–41.
Article81. DeVore S, Kohli R, Lake K, Nicholas L, Dietrich K, Balistreri WF, et al. A multidisciplinary clinical program is effective in stabilizing BMI and reducing transaminase levels in pediatric patients with NAFLD. J Pediatr Gastroenterol Nutr. 2013; 57:119–23.
Article82. Wolfenden L, Nathan NK, Sutherland R, Yoong SL, Hodder RK, Wyse RJ, et al. Strategies for enhancing the implementation of school-based policies or practices targeting risk factors for chronic disease. Cochrane Database Syst Rev. 2017; 11:CD011677.
Article83. Smith M, Hosking J, Woodward A, Witten K, MacMillan A, Field A, et al. Systematic literature review of built environment effects on physical activity and active transport: an update and new findings on health equity. Int J Behav Nutr Phys Act. 2017; 14:158.84. Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP Rep. 2021; 3:100322.
Article85. Shen J, Wong GL, Chan HL, Chan RS, Chan HY, Chu WC, et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2015; 30:139–46.
Article86. Sevastianova K, Kotronen A, Gastaldelli A, Perttila J, Hakkarainen A, Lundbom J, et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr. 2011; 94:104–11.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Should you advocate for hepatocellular carcinomasurveillance in patients with alcohol-related liverdisease or non-alcoholic fatty liver disease?
- Comparison between obese and non-obese nonalcoholic fatty liver disease
- Histologic Risk Factor for Mortality and Development of Severe Liver Disease in Biopsy-proven Non-alcoholic Fatty Liver Disease
- Are patients with alcohol-related fatty liver at increased risk of coronary heart disease?
- An analysis of polygenic risk scores for non-alcoholic fatty liver disease