Intest Res.
2022 Apr;20(2):240-250. 10.5217/ir.2021.00091.
Vedolizumab for perianal fistulizing Crohn’s disease: systematic review and meta-analysis
- Affiliations
-
- 1Inflammatory Bowel Disease Center, Section of Gastroenterology, Hepatology, and Nutrition, University of Chicago, Chicago, IL, USA
- KMID: 2529570
- DOI: http://doi.org/10.5217/ir.2021.00091
Abstract
- Background/Aims
Perianal fistulas are a debilitating manifestation of Crohn’s disease (CD). Despite the advent of anti-tumor necrosis factor (anti-TNF) therapy, the medical management of fistulizing CD continues to be challenged by unmet needs. We conducted a systematic review and meta-analysis of the effectiveness of vedolizumab for the management of perianal fistulizing CD.
Methods
A search of PubMed, EMBASE and the Cochrane Library was performed from inception to June 2020 for studies reporting rates of perianal fistula healing in CD patients treated with vedolizumab. The primary outcome of interest was complete healing of perianal fistulas and the secondary outcome was partial healing. The pooled fistula healing rates with 95% confidence intervals (CI) were calculated utilizing a random effects model.
Results
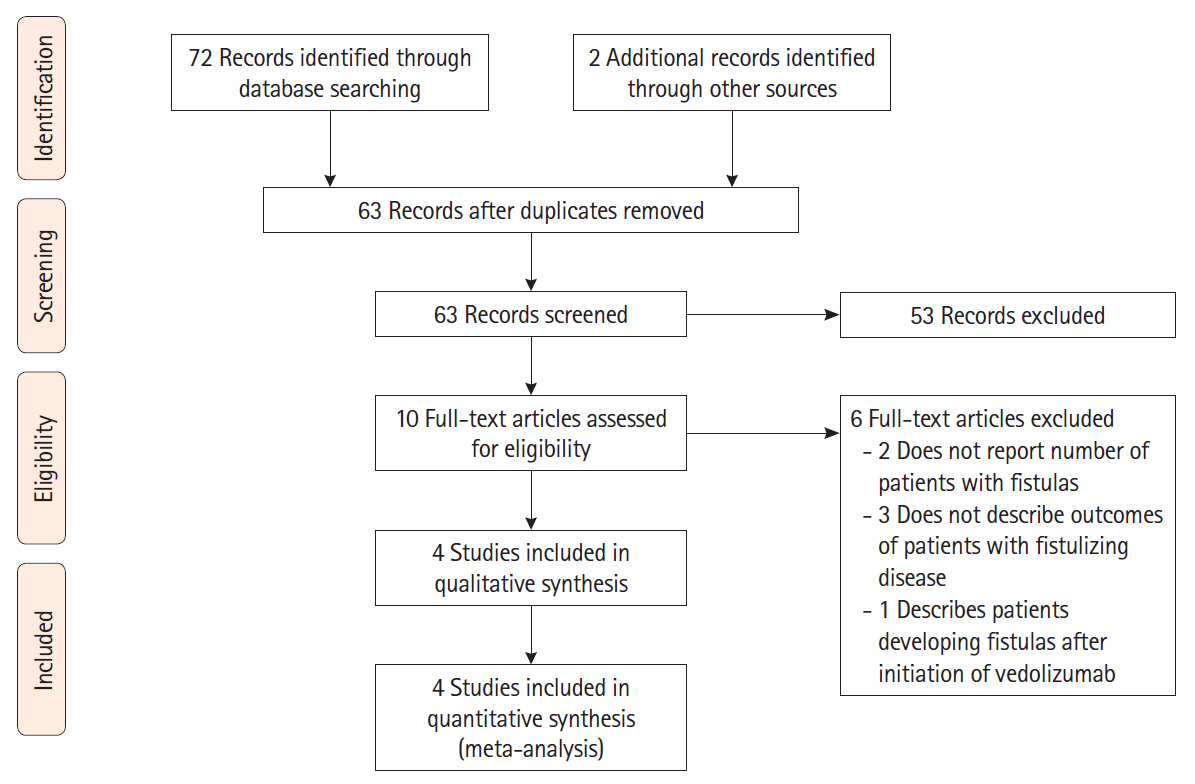
A total of 74 studies were initially identified, 4 of which met the inclusion criteria. A total of 198 patients with active perianal fistulas were included, 87% of whom had failed previous anti-TNF therapy. The pooled complete healing rate was 27.6% (95% CI, 18.9%–37.3%) with moderate heterogeneity (I2=49.4%) and the pooled partial healing rate was 34.9% (95% CI, 23.2%–47.7%) with high heterogeneity (I2=67.1%).
Conclusions
In a meta-analysis of 4 studies that included 198 patients with perianal fistulizing CD, the majority of whom had failed previous anti-TNF therapy, vedolizumab treatment led to healing of perianal fistulas in nearly one-third of the patients. The lack of high-quality data and significant study heterogeneity underscores the need for future prospective studies of fistula healing in patients receiving anti-integrin therapy.
Figure
Reference
-
1. Siegmund B, Feakins RM, Barmias G, et al. Results of the Fifth Scientific Workshop of the ECCO (II): pathophysiology of perianal fistulizing disease. J Crohns Colitis. 2016; 10:377–386.
Article2. Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002; 122:875–880.
Article3. Schwartz DA, Tagarro I, Carmen Díez M, Sandborn WJ. Prevalence of fistulizing Crohn’s disease in the United States: estimate from a systematic literature review attempt and population-based database analysis. Inflamm Bowel Dis. 2019; 25:1773–1779.
Article4. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999; 340:1398–1405.
Article5. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.
Article6. Dewint P, Hansen BE, Verhey E, et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut. 2014; 63:292–299.
Article7. West RL, van der Woude CJ, Hansen BE, et al. Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn’s disease with infliximab: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2004; 20:1329–1336.
Article8. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013; 369:711–721.
Article9. Wang Y, Wang J, Pekow J, et al. Outcome of elective switching to vedolizumab in inflammatory bowel disease patients under tumor necrosis factor antagonist-maintained clinical remission. J Gastroenterol Hepatol. 2019; 34:2090–2095.
Article10. Page MJ, Shamseer L, Tricco AC. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst Rev. 2018; 7:32.
Article11. Chapuis-Biron C, Bourrier A, Nachury M, et al. Vedolizumab for perianal Crohn’s disease: a multicentre cohort study in 151 patients. Aliment Pharmacol Ther. 2020; 51:719–727.
Article12. Schwartz D, Peyrin-Biroulet L, Lasch K, Adsul S, Danese S. A randomized double-blind phase 4 study to evaluate the safety and proportion of subjects with fistula healing in 2 dose regimens of Entyvio (vedolizumab IV) in the treatment of fistulizing Crohn’s disease (ENTERPRISE) [Internet]. c2020 [cited 2020 Jul 1]. https://clinicaltrials.gov/ct2/show/NCT02630966.13. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. c2000 [cited 2021 May 1]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Article14. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Cochrane Collaboration and John Wiley & Sons Ltd.;2019.15. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014; 72:39.
Article16. Feagan BG, Schwartz D, Danese S, et al. Efficacy of vedolizumab in fistulising Crohn’s disease: exploratory analyses of data from GEMINI 2. J Crohns Colitis. 2018; 12:621–626.
Article17. Pestour S, Nancey S, Charlois A, et al. Influence of disease location on vedolizumab effectiveness in inflammatory bowel diseases: a real-life multicenter experience. Hum Health Pathol dumas-01623835 [Preprint]. 2017; [cited 2021 May 1]. https://dumas.ccsd.cnrs.fr/dumas-01623835.18. Schwartz DA, Peyrin-Biroulet L, Lasch K, Adsul S, Danese S. 949 Efficacy and safety of 2 vedolizumab iv regimens in patients with perianal fistulizing Crohn’s disease: results of the ENTERPRISE study. Gastroenterology. 2020; 158(6 Suppl 1):S-193–S-194.
Article19. Pestour S, Nancey S, Charlois AL, et al. DOP050 Influence of disease location on vedolizumab efficacy in inflammatory bowel disease: a real-life multicentre experience. J Crohns Colitis. 2018; 12(Suppl 1):S065–S066.
Article20. Schreiber S, Lawrance IC, Thomsen OØ, Hanauer SB, Bloomfield R, Sandborn WJ. Randomised clinical trial: certolizumab pegol for fistulas in Crohn’s disease. Subgroup results from a placebo-controlled study. Aliment Pharmacol Ther. 2011; 33:185–193.
Article21. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004; 350:876–885.
Article22. Lee MJ, Parker CE, Taylor SR, et al. Efficacy of medical therapies for fistulizing Crohn’s disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018; 16:1879–1892.
Article23. Barré A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther. 2018; 47:896–905.
Article24. Feagan B. Safety and positioning of vedolizumab in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2018; 14:244–246.25. Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015; 64:77–83.
Article26. van Deventer SJ. Review article: targeting TNF alpha as a key cytokine in the inflammatory processes of Crohn’s disease. The mechanisms of action of infliximab. Aliment Pharmacol Ther. 1999; 13 Suppl 4:3–8.27. Panés J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol. 2017; 14:652–664.
Article28. Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn’s disease. World J Gastrointest Pathophysiol. 2014; 5:205–212.
Article29. Frei SM, Pesch T, Lang S, et al. A role for tumor necrosis factor and bacterial antigens in the pathogenesis of Crohn’s disease-associated fistulae. Inflamm Bowel Dis. 2013; 19:2878–2887.
Article30. Scharl M, Weber A, Fürst A, et al. Potential role for SNAIL family transcription factors in the etiology of Crohn’s disease-associated fistulae. Inflamm Bowel Dis. 2011; 17:1907–1916.
Article31. de Krijger M, Buskens CJ, Wildenberg ME, Verseijden C, de Jonge WJ, Ponsioen CY. P044 T cells expressing integrin α4β7 are abundant in fistula tracts of Crohn’s disease patients. J Crohns Colitis. 2018; 12(Suppl 1):S114.32. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006; 130:323–333.
Article33. Sandborn WJ, Feagan BG, Radford-Smith G, et al. CDP571, a humanised monoclonal antibody to tumour necrosis factor alpha, for moderate to severe Crohn’s disease: a randomised, double blind, placebo controlled trial. Gut. 2004; 53:1485–1493.
Article34. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007; 357:228–238.
Article35. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007; 146:829–838.
Article36. Harris MS, Wichary J, Zadnik M, Reinisch W. Competition for clinical trials in inflammatory bowel diseases. Gastroenterology. 2019; 157:1457–1461.
Article37. Lopez N, Ramamoorthy S, Sandborn WJ. Recent advances in the management of perianal fistulizing Crohn’s disease: lessons for the clinic. Expert Rev Gastroenterol Hepatol. 2019; 13:563–577.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidentally Detected Asymptomatic Perianal Abscess in an Adolescent during Crohn's Disease Diagnosis: Is Routine Pelvic Imaging Required in Korean Pediatric Patients at Diagnosis?
- Surgical treatment of perianal fistula in Crohn's disease
- Efficacy of Infliximab in the Treatment of Korean Patients with Crohn's Disease
- A Case of Crohn's Disease Showing a Skin Lesion with a Cobblestone-like Appearance in the Perianal Region
- An Introduction of the Systematic Review and Meta-Analysis