J Korean Med Sci.
2022 Apr;37(16):e123. 10.3346/jkms.2022.37.e123.
The Comparative Risk of Serious Adverse Events With Tofacitinib and TNF Inhibitors in Patients With Ulcerative Colitis: The Korean Experience as Revealed by a National Database
- Affiliations
-
- 1Healthcare Review and Assessment Committee, Health Insurance Review and Assessment Service, Wonju, Korea
- 2Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2529291
- DOI: http://doi.org/10.3346/jkms.2022.37.e123
Abstract
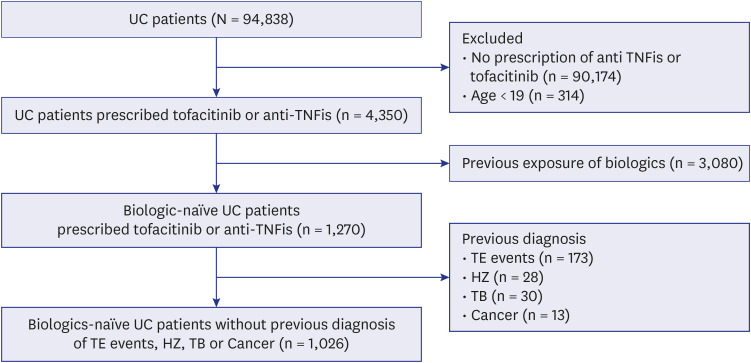
- Tofacitinib is an oral, small-molecule Janus kinase inhibitor approved in South Korea for the treatment of moderate to severe ulcerative colitis (UC) on May 1, 2019. However, safety data are lacking. We investigated the incidence of serious adverse events (SAEs) in patients with UC using tofacitinib from the National Health Insurance Service database. In all, 1,026 UC patients were enrolled in this study. The overall incidences (100 person-years; 95% confidence interval) of SAEs were 4.06 (1.63–8.36) and 6.30 (4.59–8.43) in the tofacitinib and anti-TNFi groups, respectively. No thromboembolic event occurred and major cardiovascular events occurred in only three patients (two unstable angina and one congestive heart failure) in the tofacitinib group. The incidence of herpes zoster and tuberculosis did not differ between the two groups. There was no difference in the overall incidence of SAEs, including thromboembolic events, between tofacitinib- and TNFi-treated UC patients.
Keyword
Figure
Reference
-
1. Lee JY, Oh K, Hong HS, Kim K, Hong SW, Park JH, et al. Risk and characteristics of tuberculosis after anti-tumor necrosis factor therapy for inflammatory bowel disease: a hospital-based cohort study from Korea. BMC Gastroenterol. 2021; 21(1):390. PMID: 34670529.2. Yen HH, Weng MT, Tung CC, Wang YT, Chang YT, Chang CH, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019; 17(1):54–62. PMID: 30449079.3. Hong SW, Ye BD. The first step to unveil the epidemiology of inflammatory bowel disease in Central Asia. Intest Res. 2020; 18(4):345–346. PMID: 33131230.4. Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012; 367(7):616–624. PMID: 22894574.5. Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376(18):1723–1736. PMID: 28467869.6. Sandborn WJ, Panés J, D’Haens GR, Sands BE, Su C, Moscariello M, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019; 17(8):1541–1550. PMID: 30476584.7. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010; 375(9715):657–663. PMID: 20149425.8. Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011; 60(7):937–943. PMID: 21339206.9. Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014; 146(3):835–848.e6. PMID: 24462530.10. Chung WS, Lin CL, Hsu WH, Kao CH. Inflammatory bowel disease increases the risks of deep vein thrombosis and pulmonary embolism in the hospitalized patients: a nationwide cohort study. Thromb Res. 2015; 135(3):492–496. PMID: 25596768.11. Weng MT, Park SH, Matsuoka K, Tung CC, Lee JY, Chang CH, et al. Incidence and risk factor analysis of thromboembolic events in East Asian patients with inflammatory bowel disease, a multinational collaborative study. Inflamm Bowel Dis. 2018; 24(8):1791–1800. PMID: 29726897.12. Liu J, Gao X, Chen Y, Mei Q, Zhu L, Qian J, et al. Incidence and risk factors for venous thrombosis among patients with inflammatory bowel disease in China: a multicenter retrospective study. Intest Res. 2021; 19(3):313–322. PMID: 33232589.13. Jeon D. Latent tuberculosis infection: recent progress and challenges in South Korea. Korean J Intern Med. 2020; 35(2):269–275. PMID: 32131570.14. Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020; 6(3):e001395. PMID: 33127856.15. Kim HJ, Hann HJ, Hong SN, Kim KH, Ahn IM, Song JY, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21(3):623–630. PMID: 25647154.16. Curtis JR, Regueiro M, Yun H, Su C, DiBonaventura M, Lawendy N, et al. Tofacitinib treatment safety in moderate to severe ulcerative colitis: comparison of observational population cohort data from the IBM MarketScan® administrative claims database with tofacitinib trial data. Inflamm Bowel Dis. 2021; 27(9):1394–1408. PMID: 33324993.17. Sinagra E, Perricone G, Romano C, Cottone M. Heart failure and anti tumor necrosis factor-alpha in systemic chronic inflammatory diseases. Eur J Intern Med. 2013; 24(5):385–392. PMID: 23333028.18. Sandborn WJ, Panés J, Sands BE, Reinisch W, Su C, Lawendy N, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019; 50(10):1068–1076. PMID: 31599001.19. Deepak P, Alayo QA, Khatiwada A, Lin B, Fenster M, Dimopoulos C, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19(8):1592–1601.e3. PMID: 32629130.20. Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142(2):257–265.e1-3. PMID: 22062358.21. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146(1):85–95. PMID: 23735746.22. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146(1):96–109.e1. PMID: 23770005.23. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353(23):2462–2476. PMID: 16339095.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current and Emerging Biologics for Ulcerative Colitis
- Effects of Dextran Sulfate Sodium-Induced Ulcerative Colitis on the Disposition of Tofacitinib in Rats
- The Efficacy and Safety of a Tofacitinib in the Treatment of Active Ulcerative Colitis
- Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies