Endocrinol Metab.
2022 Apr;37(2):333-343. 10.3803/EnM.2021.1202.
Association between Elevated Plasma Homocysteine and Low Skeletal Muscle Mass in Asymptomatic Adults
- Affiliations
-
- 1Department of Physical and Rehabilitation Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Biostatistics, Department of R&D Management, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Medical Research Institute, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2529225
- DOI: http://doi.org/10.3803/EnM.2021.1202
Abstract
- Background
Homocysteine has been drawing attention with a closed linkage with skeletal muscle. However, the association of hyperhomocysteinemia with decreased skeletal muscle mass remains unclear. We aimed to investigate the association of hyperhomocysteinemia with low skeletal muscle mass (LMM) in asymptomatic adults.
Methods
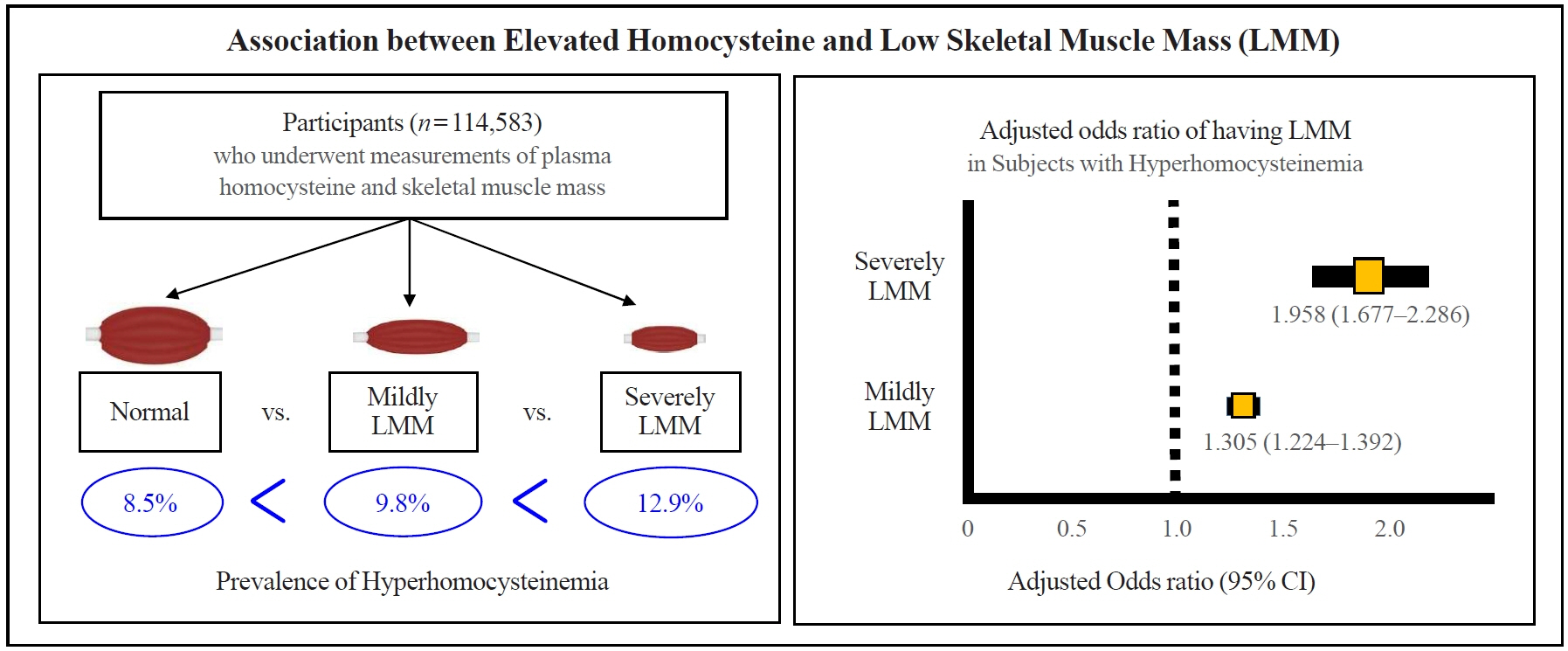
This was a cross-sectional study of 114,583 community-dwelling adults without cancer, stroke, or cardiovascular diseases who underwent measurements of plasma homocysteine and body composition analysis from 2012 to 2018. Hyperhomocysteinemia was defined as >15 μmol/L. Skeletal muscle mass index (SMI) was calculated based on appendicular muscle mass (kg)/height (m)2. Participants were classified into three groups based on SMI: “normal,” “mildly low,” and “severely low.”
Results
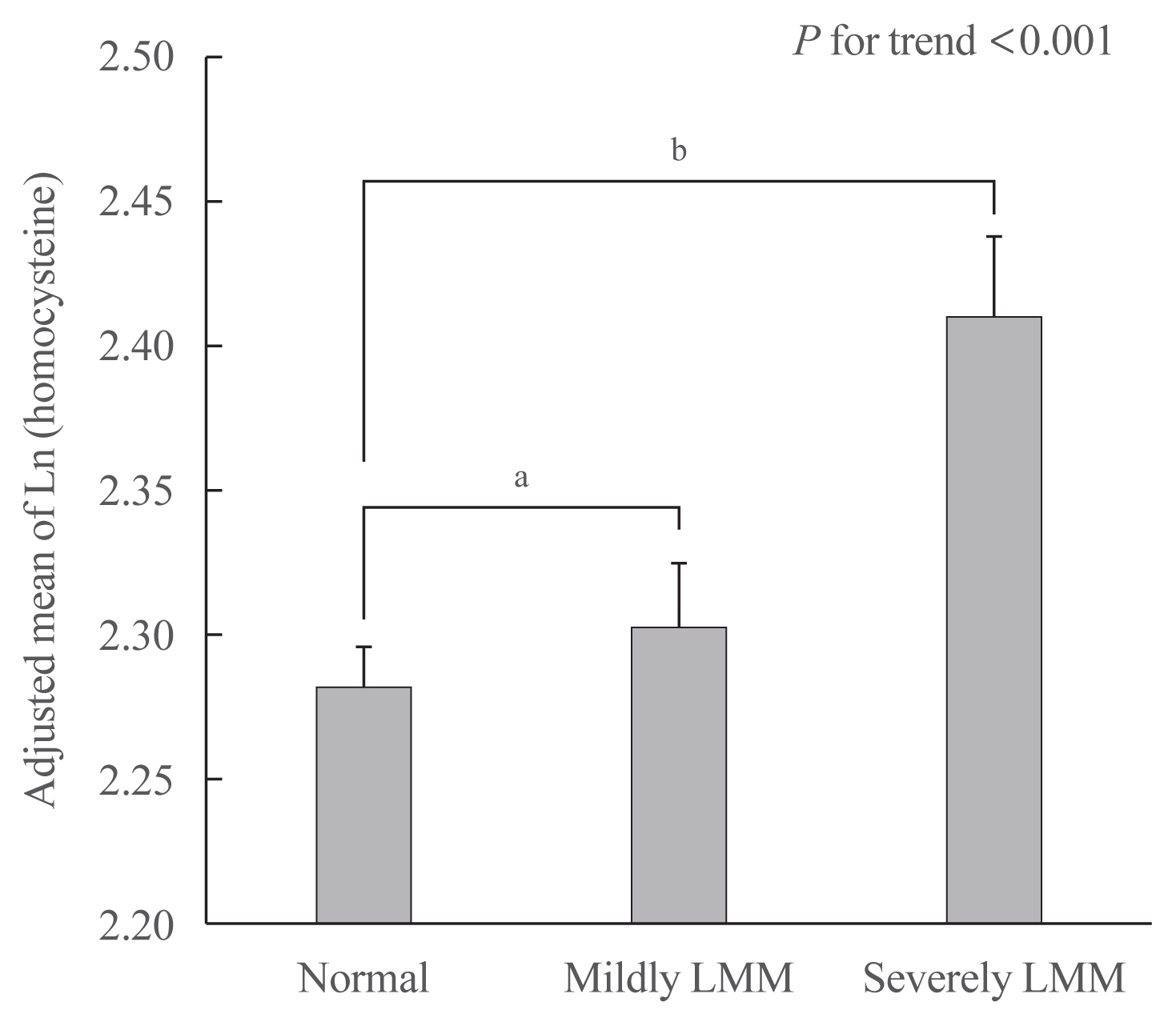
The prevalence of hyperhomocysteinemia was the highest in subjects with severely LMM (12.9%), followed by those with mildly LMM (9.8%), and those with normal muscle mass (8.5%) (P for trend <0.001). In a multivariable logistic regression model, hyperhomocysteinemia was significantly associated with having a mildly LMM (odds ratio [OR], 1.305; 95% confidence interval [CI], 1.224 to 1.392) and severely LMM (OR, 1.958; 95% CI, 1.667 to 2.286), respectively. One unit increment of log-transformed homocysteine was associated with 1.360 and 2.169 times higher risk of having mildly LMM and severely LMM, respectively.
Conclusion
We demonstrated that elevated homocysteine has an independent association with LMM in asymptomatic adults, supporting that hyperhomocysteinemia itself can be a risk for decline in skeletal musculature.
Keyword
Figure
Cited by 1 articles
-
Differences in Type 2 Fiber Composition in the Vastus Lateralis and Gluteus Maximus of Patients with Hip Fractures
Jingwen Tian, Minchul Song, Kyu Jeong Cho, Ho Yeop Lee, Sang Hyeon Ju, Jung Ryul Lim, Ha Thi Nga, Thi Linh Nguyen, Ji Sun Moon, Hyo Ju Jang, Jung-Mo Hwang, Hyon-Seung Yi
Endocrinol Metab. 2024;39(3):521-530. doi: 10.3803/EnM.2024.1935.
Reference
-
1. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus date on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21:300–7.2. Woo N, Kim SH. Sarcopenia influences fall-related injuries in community-dwelling older adults. Geriatr Nurs. 2014; 35:279–82.
Article3. Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc. 2014; 15:551–8.
Article4. Szulc P, Munoz F, Marchand F, Chapurlat R, Delmas PD. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr. 2010; 91:1227–36.
Article5. Srikanthan P, Horwich TB, Tseng CH. Relation of muscle mass and fat mass to cardiovascular disease mortality. Am J Cardiol. 2016; 117:1355–60.
Article6. Abe T, Iwata K, Yoshimura Y, Shinoda T, Inagaki Y, Ohya S, et al. Low muscle mass is associated with walking function in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020; 29:105259.
Article7. Attaway AH, Welch N, Hatipoglu U, Zein JG, Dasarathy S. Muscle loss contributes to higher morbidity and mortality in COPD: an analysis of national trends. Respirology. 2021; 26:62–71.
Article8. Xiao J, Caan BJ, Cespedes Feliciano EM, Meyerhardt JA, Peng PD, Baracos VE, et al. Association of low muscle mass and low muscle radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surg. 2020; 155:942–9.
Article9. Kim H, Hirano H, Edahiro A, Ohara Y, Watanabe Y, Kojima N, et al. Sarcopenia: prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr Gerontol Int. 2016; 16(Suppl 1):110–22.
Article10. Tinelli C, Di Pino A, Ficulle E, Marcelli S, Feligioni M. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front Nutr. 2019; 6:49.
Article11. Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res. 2018; 41:372–83.
Article12. van Schoor NM, Swart KM, Pluijm SM, Visser M, Simsek S, Smulders Y, et al. Cross-sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr. 2012; 66:174–81.
Article13. Wang CS, Wong TC, Duong TV, Su CT, Chen HH, Chen TH, et al. Hyperhomocysteinemia associated with low muscle mass, muscle function in elderly hemodialysis patients: an analysis of multiple dialysis centers. Biomed Res Int. 2019; 2019:9276097.
Article14. Vidoni ML, Pettee Gabriel K, Luo ST, Simonsick EM, Day RS. Relationship between homocysteine and muscle strength decline: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2018; 73:546–51.
Article15. Lee WJ, Peng LN, Loh CH, Chen LK. Sex-different associations between serum homocysteine, high-sensitivity C-reactive protein and sarcopenia: results from I-Lan Longitudinal Aging Study. Exp Gerontol. 2020; 132:110832.
Article16. Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygard O, et al. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr. 2008; 88:738–46.
Article17. Atkins JL, Whincup PH, Morris RW, Wannamethee SG. Low muscle mass in older men: the role of lifestyle, diet and cardiovascular risk factors. J Nutr Health Aging. 2014; 18:26–33.
Article18. Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012; 24:623–7.
Article19. Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998; 338:1–7.
Article20. Kurose S, Nishikawa S, Nagaoka T, Kusaka M, Kawamura J, Nishioka Y, et al. Prevalence and risk factors of sarcopenia in community-dwelling older adults visiting regional medical institutions from the Kadoma Sarcopenia Study. Sci Rep. 2020; 10:19129.
Article21. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019; 393:2636–46.
Article22. Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002; 288:2015–22.23. Wang CY, Chen ZW, Zhang T, Liu J, Chen SH, Liu SY, et al. Elevated plasma homocysteine level is associated with ischemic stroke in Chinese hypertensive patients. Eur J Intern Med. 2014; 25:538–44.
Article24. Anjanappa M, Corden M, Green A, Roberts D, Hoskin P, McWilliam A, et al. Sarcopenia in cancer: risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020; 16:50–7.
Article25. Bozzetti F. Chemotherapy-induced sarcopenia. Curr Treat Options Oncol. 2020; 21:7.
Article26. Devaux M, Sassi F. Alcohol consumption and harmful drinking: trends and social disparities across OECD countries. OECD Health Work Pap. 2015. 79. Available from: https://doi.org/10.1787/5js1qwkz2p9s-en .
Article27. Park CH, Do JG, Lee YT, Yoon KJ. Sarcopenic obesity associated with high-sensitivity C-reactive protein in age and sex comparison: a two-center study in South Korea. BMJ Open. 2018; 8:e021232.
Article28. World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization;2020.29. Kang SS, Wong PW, Malinow MR. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr. 1992; 12:279–98.
Article30. Abai B. StatPearls. Treasure Island: StatPearls Publishing;2021. Chapter, Hyperhomocysteinemia [cited 2021 Dec 21]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554408 .31. Hasan T, Arora R, Bansal AK, Bhattacharya R, Sharma GS, Singh LR. Disturbed homocysteine metabolism is associated with cancer. Exp Mol Med. 2019; 51:1–13.
Article32. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002; 50:889–96.
Article33. Mijnarends DM, Schols JM, Meijers JM, Tan FE, Verlaan S, Luiking YC, et al. Instruments to assess sarcopenia and physical frailty in older people living in a community (care) setting: similarities and discrepancies. J Am Med Dir Assoc. 2015; 16:301–8.
Article34. Brustolin S, Giugliani R, Felix TM. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010; 43:1–7.
Article35. Majumder A, Behera J, Jeremic N, Tyagi SC. Hypermethylation: causes and consequences in skeletal muscle myopathy. J Cell Biochem. 2017; 118:2108–17.
Article36. Swart KM, Enneman AW, van Wijngaarden JP, van Dijk SC, Brouwer-Brolsma EM, Ham AC, et al. Homocysteine and the methylenetetrahydrofolate reductase 677C→T polymorphism in relation to muscle mass and strength, physical performance and postural sway. Eur J Clin Nutr. 2013; 67:743–8.
Article37. Veeranki S, Lominadze D, Tyagi SC. Hyperhomocysteinemia inhibits satellite cell regenerative capacity through p38 alpha/beta MAPK signaling. Am J Physiol Heart Circ Physiol. 2015; 309:H325–34.
Article38. Veeranki S, Winchester LJ, Tyagi SC. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochim Biophys Acta. 2015; 1852:732–41.
Article39. Singh M, George AK, Eyob W, Homme RP, Stansic D, Tyagi SC. High-methionine diet in skeletal muscle remodeling: epigenetic mechanism of homocysteine-mediated growth retardation. Can J Physiol Pharmacol. 2021; 99:56–63.
Article40. Dierkes J, Jeckel A, Ambrosch A, Westphal S, Luley C, Boeing H. Factors explaining the difference of total homocysteine between men and women in the European Investigation Into Cancer and Nutrition Potsdam study. Metabolism. 2001; 50:640–5.
Article41. Ostrakhovitch EA, Tabibzadeh S. Homocysteine and age-associated disorders. Ageing Res Rev. 2019; 49:144–64.
Article42. Sobczak A, Wardas W, Zielinska-Danch W, Pawlicki K. The influence of smoking on plasma homocysteine and cysteine levels in passive and active smokers. Clin Chem Lab Med. 2004; 42:408–14.
Article43. Gibson A, Woodside JV, Young IS, Sharpe PC, Mercer C, Patterson CC, et al. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers: a randomized, crossover intervention study. QJM. 2008; 101:881–7.
Article44. Petersen AM, Magkos F, Atherton P, Selby A, Smith K, Rennie MJ, et al. Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab. 2007; 293:E843–8.
Article45. Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. 2013; 14:15074–91.
Article46. Yoo SZ, No MH, Heo JW, Park DH, Kang JH, Kim SH, et al. Role of exercise in age-related sarcopenia. J Exerc Rehabil. 2018; 14:551–8.
Article47. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31.
Article48. Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, van Kan GA, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012; 3:181–90.
Article49. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001; 69:89–95.50. Tosato M, Marzetti E, Cesari M, Savera G, Miller RR, Bernabei R, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res. 2017; 29:19–27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Daily Calcium Intake and Plasma Homocysteine Concentrations in Adults
- Amount of Daily Protein Intake Is Not Associated with Skeletal Muscle Strength in Older Adults
- Measurement of the Calf Muscle Circumference is Useful for Diagnosing Sarcopenia in Older Adults Requiring Long-Term Care
- The Significance of Plasma Homocysteine Level in Pregnant Women with Severe Preeclampsia
- Association between N-Terminal Prohormone Brain Natriuretic Peptide and Decreased Skeletal Muscle Mass in a Healthy Adult Population: A Cross-Sectional Study