Cancer Res Treat.
2022 Apr;54(2):458-468. 10.4143/crt.2021.425.
Histone Deacetylase as a Valuable Predictive Biomarker and Therapeutic Target in Immunotherapy for Non-Small Cell Lung Cancer
- Affiliations
-
- 1Cancer Research Institute, Korea University College of Medicine, Seoul, Korea
- 2Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea
- 3Department of Pathology, Korea University Guro Hospital, Seoul, Korea
- KMID: 2528215
- DOI: http://doi.org/10.4143/crt.2021.425
Abstract
- Purpose
Histone deacetylase inhibitors (HDACis) are epigenetic regulators and used clinically for hematopoietic malignancies. Recently, HDACis have received attention as a factor that modulates the immune system. In this study, the role of histone deacetylase (HDAC) expression as a predictive marker in lung cancer patients who were treated with immune checkpoint inhibitors (ICIs) and the role of HDACi and ICI combination treatment in the mouse tumor model were analyzed.
Materials and Methods
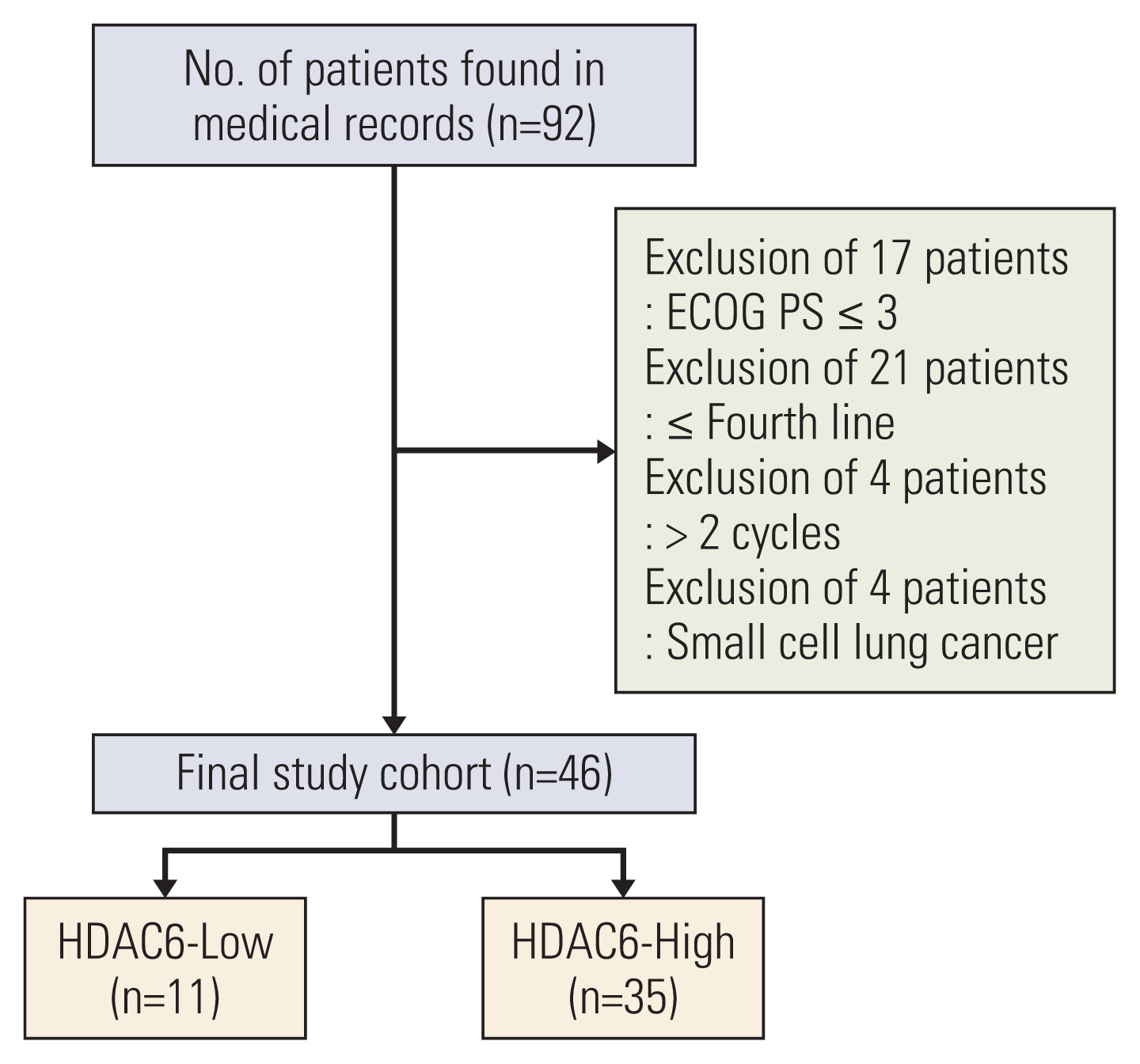
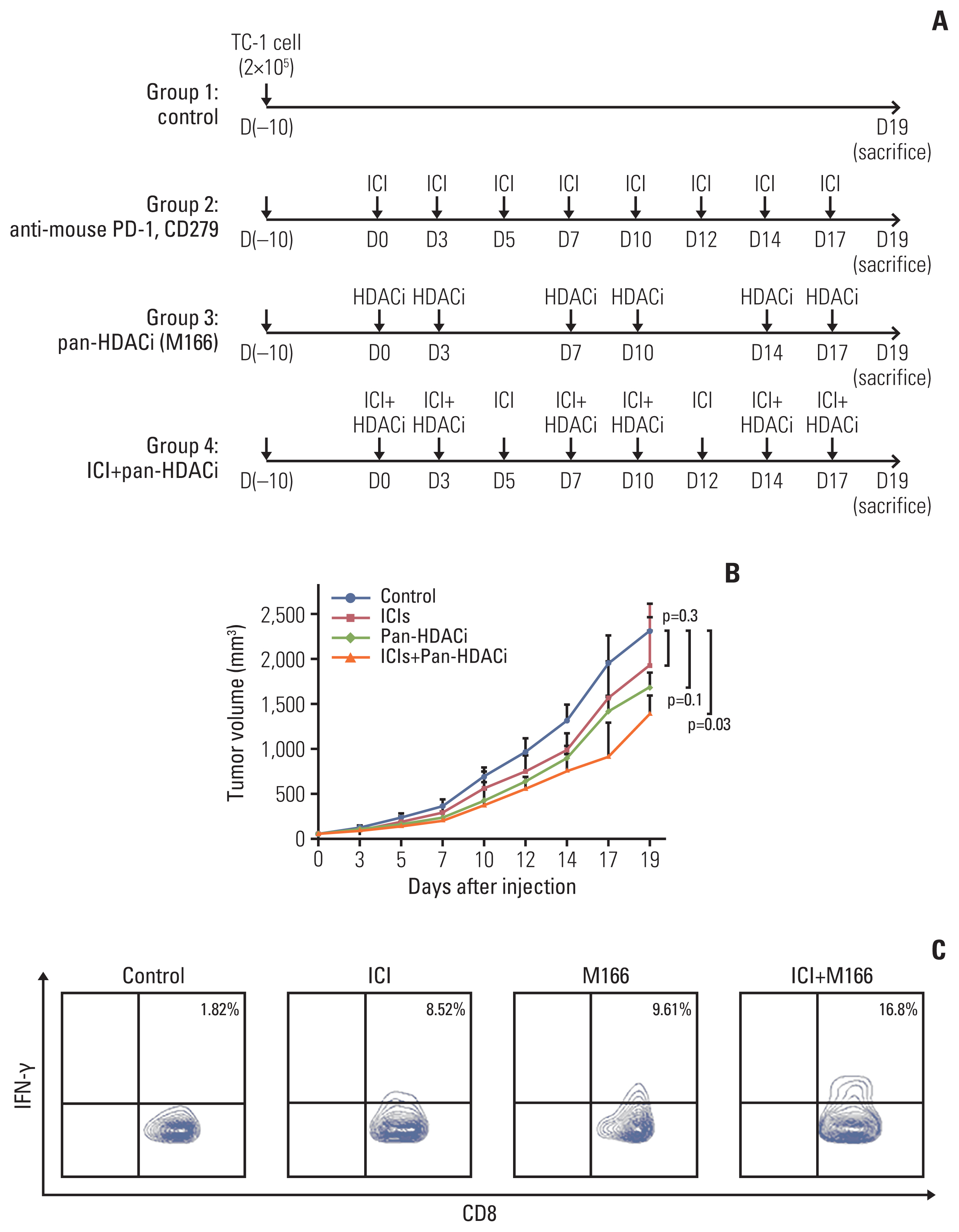
The overall response rate (ORR) and progression-free survival (PFS) were analyzed by the expression of HDAC. In vitro assay, the mRNA and protein expression levels of cytokines and programmed death-ligand 1 (PD-L1) were analyzed after HDACi treatment. In vivo assay, TC-1 tumor-bearing mice were treated with HDACi and mouse programmed cell death 1 (PD-1) inhibitor.
Results
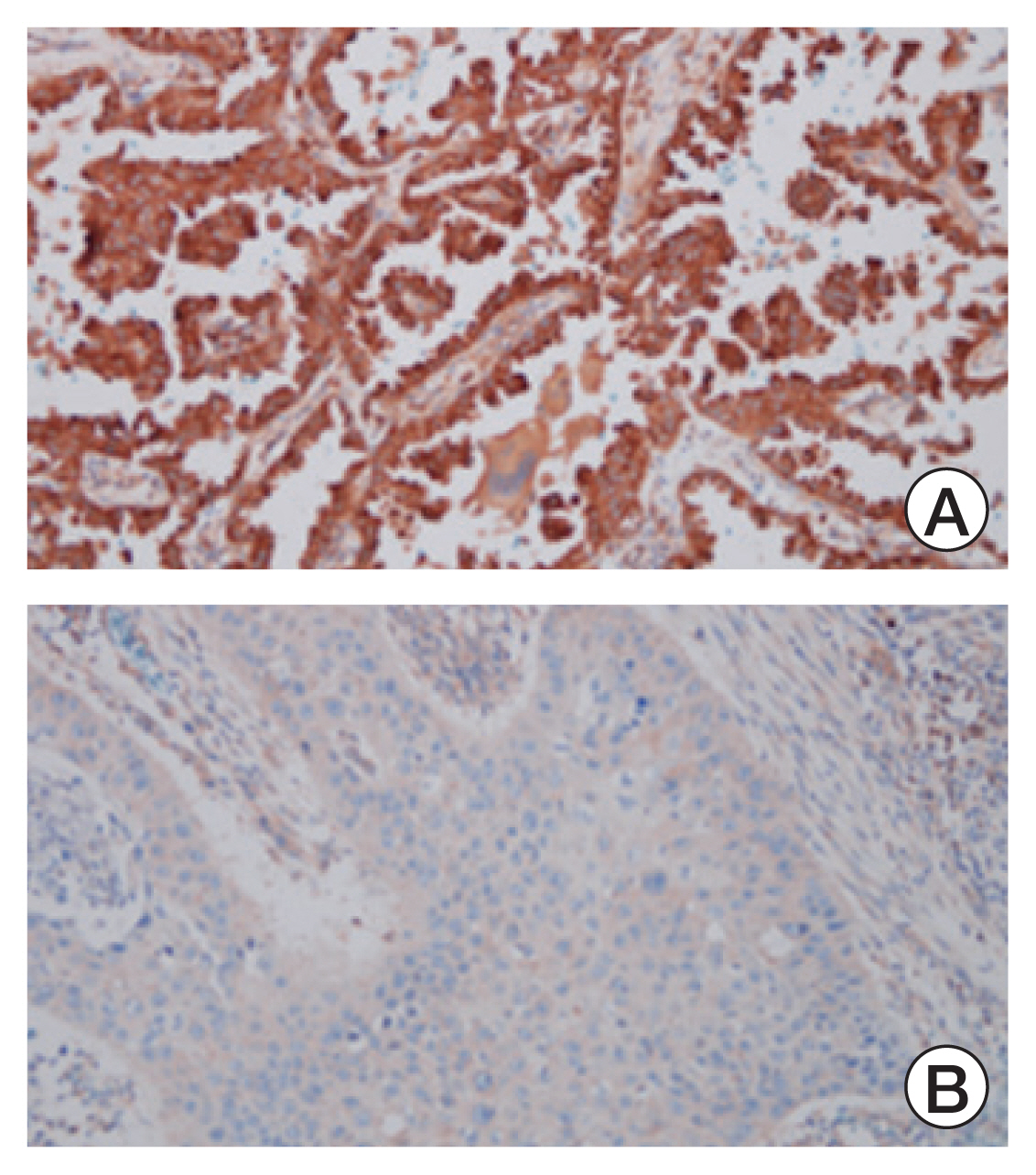
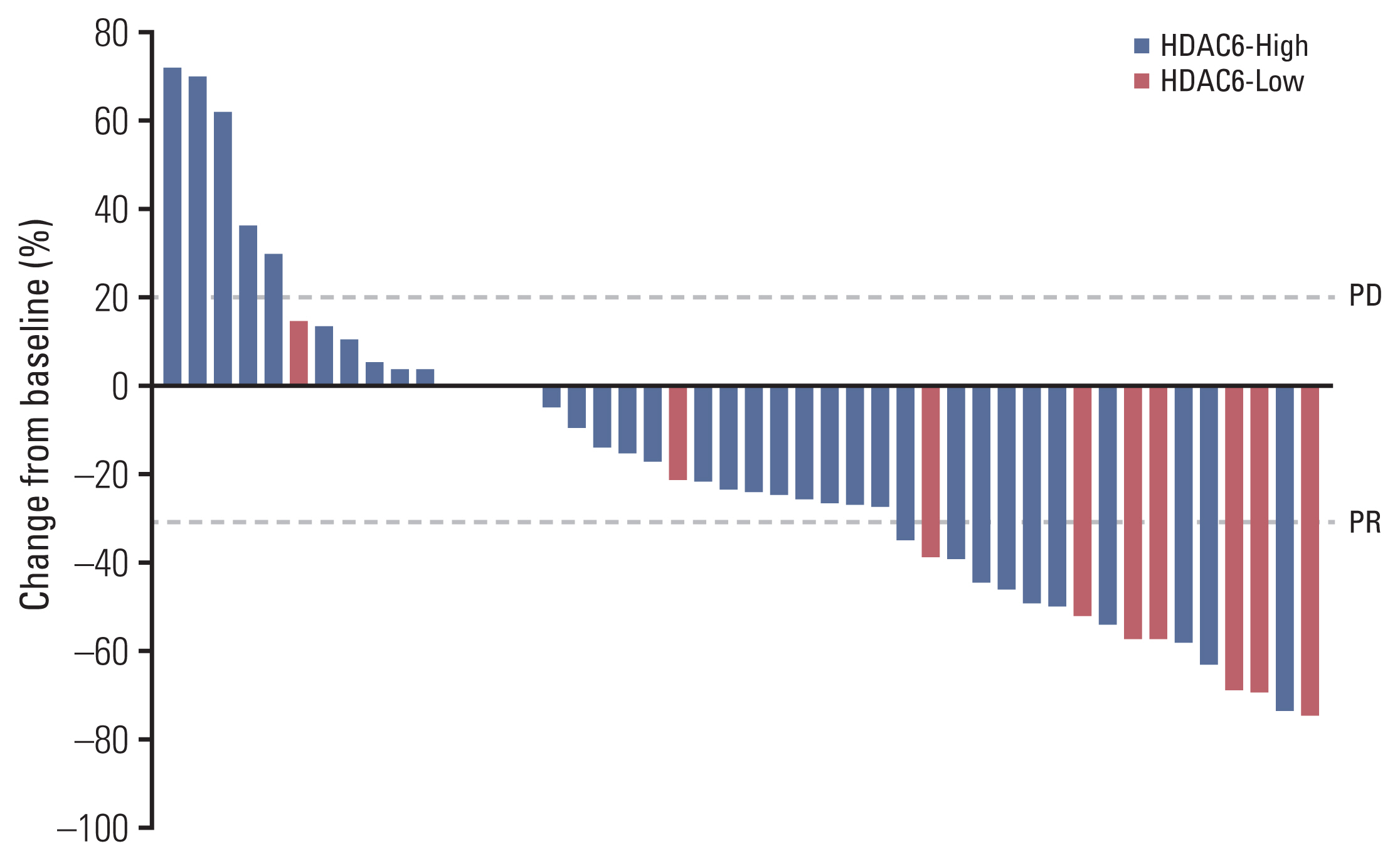
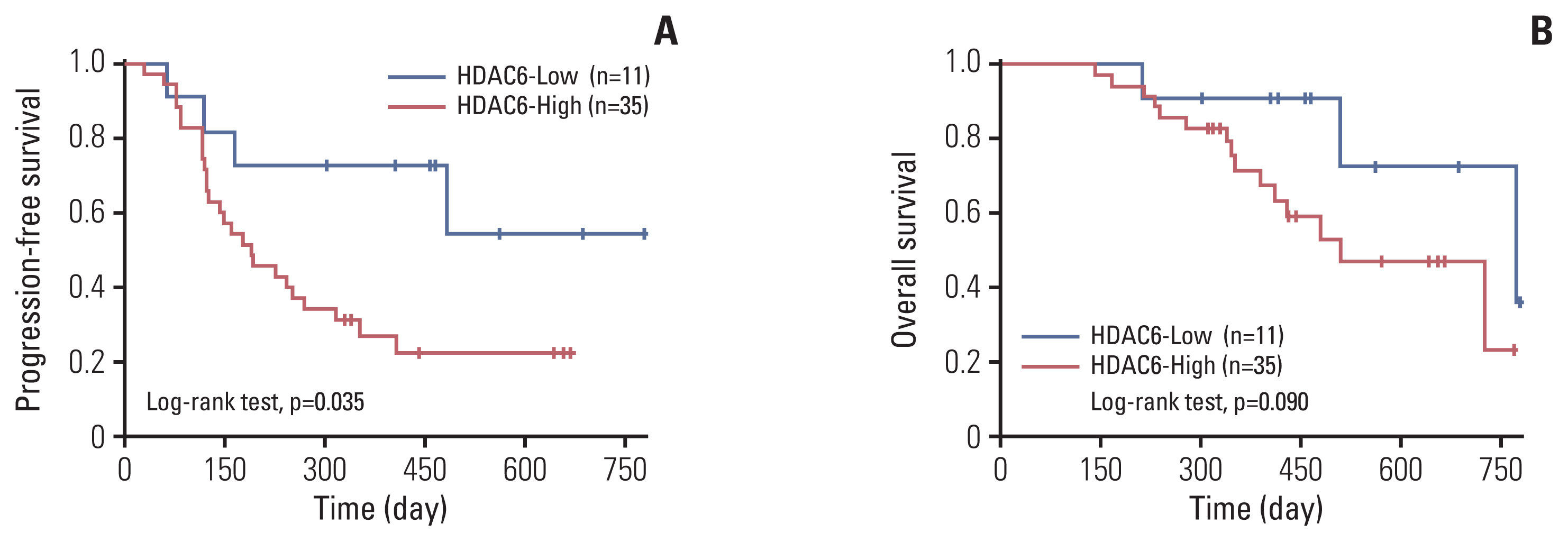
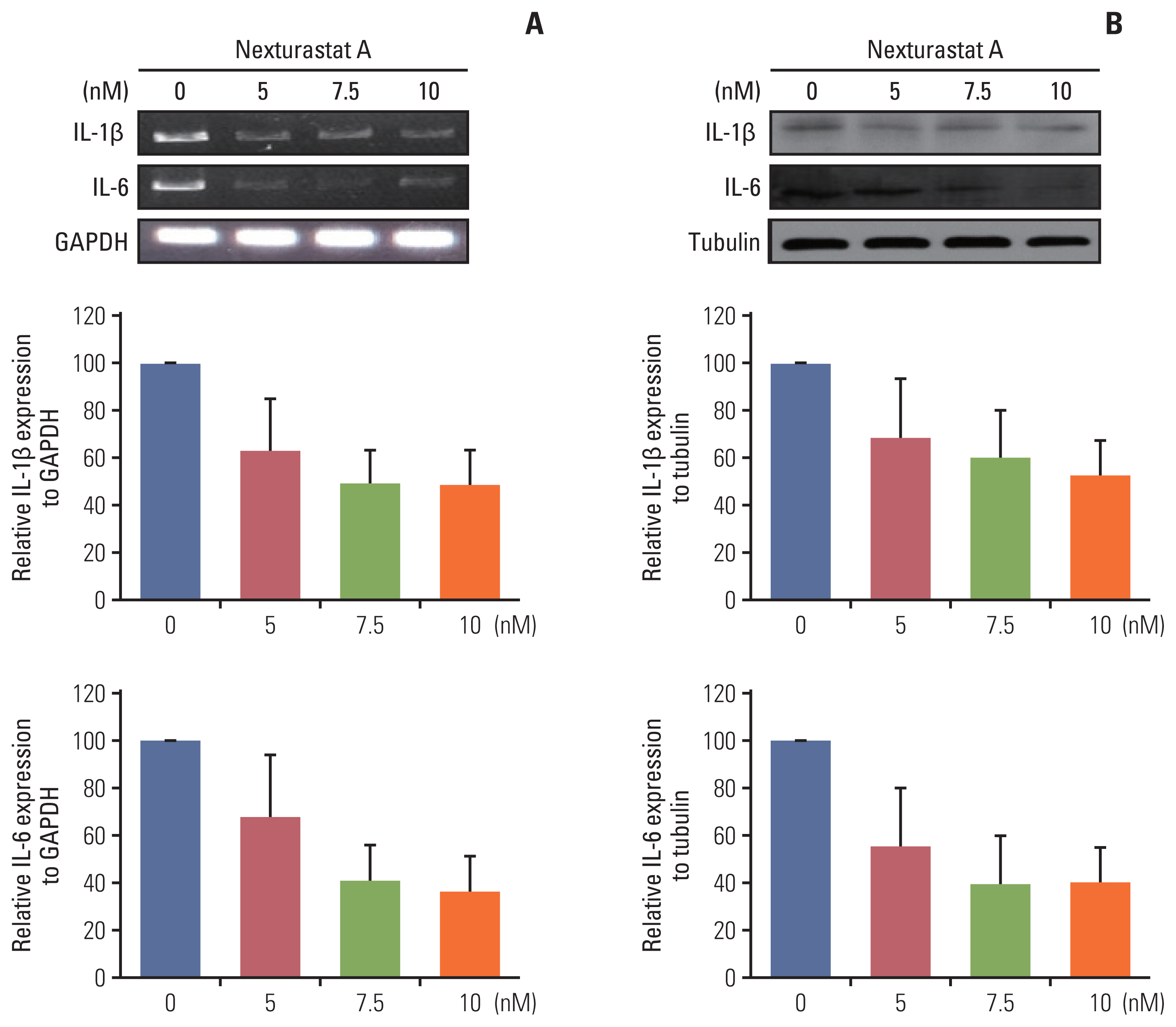
The HDAC6 low expression group showed high ORR and prolonged PFS. When the selective HDAC6 inhibitor was administered to the A549 cell line, the levels of interleukin-1β and interleukin-6 decreased and the expression of PD-L1 was reduced. Mice that received both the mouse PD-1 inhibitor and pan-HDACi had a smaller tumor size than that of the mice from the control group. Moreover, mice treated with the mouse PD-1 inhibitor and pan-HDACi generated greater numbers of E7-specific CD8+ T cells.
Conclusion
HDAC6 expression can predict the prognosis of non–small cell lung cancerpatients who were treated with ICIs. Furthermore, co-treatment with HDACi and PD-1 inhibitor was shown to decrease the tumor growth rate and create a favorable tumor microenvironment for cytotoxic T lymphocytes in the TC-1 mouse model.
Keyword
Figure
Reference
-
References
1. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016; 375:1767–78.
Article2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018; 378:158–68.
Article3. Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016; 28:383–91.
Article4. Law AM, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. 2020; 9:561.
Article5. Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel). 2016; 4:28.
Article6. Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013; 288:36691–702.
Article7. Messerschmidt JL, Prendergast GC, Messerschmidt GL. How cancers escape immune destruction and mechanisms of action for the new significantly active immune therapies: helping nonimmunologists decipher recent advances. Oncologist. 2016; 21:233–43.
Article8. Wang X, Waschke BC, Woolaver RA, Chen Z, Zhang G, Piscopio AD, et al. Histone deacetylase inhibition sensitizes PD1 blockade-resistant B-cell lymphomas. Cancer Immunol Res. 2019; 7:1318–31.
Article9. Gryder BE, Sodji QH, Oyelere AK. Targeted cancer therapy: giving histone deacetylase inhibitors all they need to succeed. Future Med Chem. 2012; 4:505–24.
Article10. Hull EE, Montgomery MR, Leyva KJ. HDAC Inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. Biomed Res Int. 2016; 2016:8797206.
Article11. Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018; 18:309–24.12. Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996; 56:21–6.13. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–33.
Article14. Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016; 4:48.
Article15. Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest. 2018; 128:3209–18.
Article16. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253–68.
Article17. Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis. 2015; 36:1085–93.
Article18. Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018; 281:57–61.
Article19. Schenk KM, Reuss JE, Choquette K, Spira AI. A review of canakinumab and its therapeutic potential for non-small cell lung cancer. Anticancer Drugs. 2019; 30:879–85.
Article20. Li J, Xu J, Yan X, Jin K, Li W, Zhang R. Targeting interleukin-6 (IL-6) sensitizes anti-PD-L1 treatment in a colorectal cancer preclinical model. Med Sci Monit. 2018; 24:5501–8.
Article21. Carta S, Tassi S, Semino C, Fossati G, Mascagni P, Dinarello CA, et al. Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: role of microtubules. Blood. 2006; 108:1618–26.22. Tang J, Pearce L, O’Donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno-oncology landscape. Nat Rev Drug Discov. 2018; 17:783–4.
Article23. Hontecillas-Prieto L, Flores-Campos R, Silver A, de Alava E, Hajji N, Garcia-Dominguez DJ. Synergistic enhancement of cancer therapy using HDAC inhibitors: opportunity for clinical trials. Front Genet. 2020; 11:578011.
Article24. Li Y, Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med. 2016; 6:a026831.
Article25. Knox T, Sahakian E, Banik D, Hadley M, Palmer E, Noonepalle S, et al. Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Sci Rep. 2019; 9:6136.
Article26. Laino AS, Betts BC, Veerapathran A, Dolgalev I, Sarnaik A, Quayle SN, et al. HDAC6 selective inhibition of melanoma patient T-cells augments anti-tumor characteristics. J Immunother Cancer. 2019; 7:33.
Article27. Banik D, Moufarrij S, Villagra A. Immunoepigenetics combination therapies: an overview of the role of HDACs in cancer immunotherapy. Int J Mol Sci. 2019; 20:2241.
Article28. Moufarrij S, Srivastava A, Gomez S, Hadley M, Palmer E, Austin PT, et al. Combining DNMT and HDAC6 inhibitors increases anti-tumor immune signaling and decreases tumor burden in ovarian cancer. Sci Rep. 2020; 10:3470.
Article29. Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009; 280:168–76.
Article30. Deng S, Hu Q, Zhang H, Yang F, Peng C, Huang C. HDAC3 inhibition upregulates PD-L1 expression in B-cell lymphomas and augments the efficacy of anti-PD-L1 therapy. Mol Cancer Ther. 2019; 18:900–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- Immunotherapy for Non-Small Cell Lung Cancer
- Formoxanthone C Inhibits Malignant Tumor Phenotypes of Human A549 Multidrug Resistant-cancer Cells through Signal Transducer and Activator of Transcription 1-Histone Deacetylase 4 Signaling
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics
- New Promising Therapeutic Modalities for Thyroid Cancers: Histone Deacetylase Inhibitor, PPAR-gamma Agonist, and Retinoic Acid