J Korean Med Sci.
2022 Apr;37(15):e116. 10.3346/jkms.2022.37.e116.
Autoimmune Hepatitis Following Vaccination for SARS-CoV-2 in Korea: Coincidence or Autoimmunity?
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 2Department of Internal Medicine, Inje University College of Medicine, Seoul, Korea
- 3Regeneration Medicine Research Center, Yonsei University Wonju College of Medicine, Wonju, Korea
- 4Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea
- KMID: 2528174
- DOI: http://doi.org/10.3346/jkms.2022.37.e116
Abstract
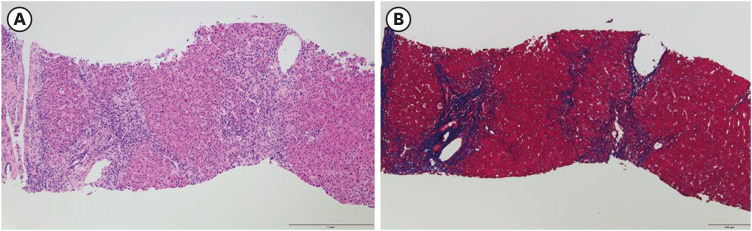
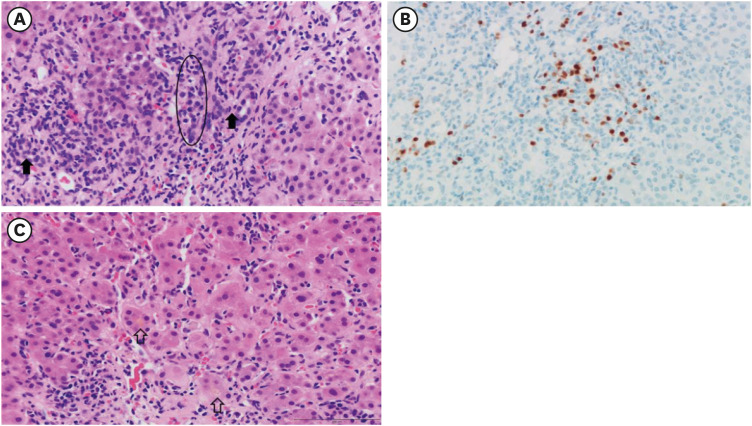
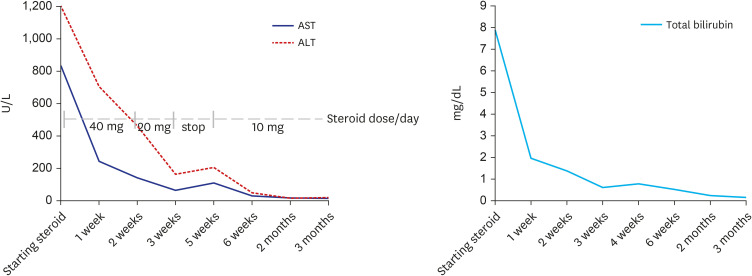
- Autoimmune hepatitis (AIH) is a chronic, autoimmune disease of the liver that occurs when the body’s immune system attacks liver cells, causing the liver to be inflamed. AIH is one of the manifestations of a coronavirus disease 2019 (COVID-19), as well as an adverse event occurring after vaccination against severe acute respiratory syndrome coronavirus 2 (SARSCoV-2). Few cases of AIH have been described after vaccination with two messenger RNA (mRNA)-based vaccines—BTN162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna)—against SARS-CoV-2. Herein, we report a case of AIH occurring after Pfizer-BioNTech COVID-19 vaccine. A 27-year-old female presented with jaundice and hepatomegaly, appearing 14 days after receiving the second dose of Pfizer-BioNTech vaccine. Her laboratory results showed abnormal liver function with high total immunoglobulin G level. She was diagnosed with AIH with histologic finding and successfully treated with oral prednisolone. We report an AIH case after COVID-19 vaccination in Korea.
Figure
Reference
-
1. World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance 2020. Updated 2020. Accessed November 28, 2021. https://apps.who.int/iris/handle/10665/331506 .2. Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021; 33(2):155–162. PMID: 33332890.3. Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021; 75(1):222–224. PMID: 33862041.4. Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021; 96(5):534–537. PMID: 33606296.5. Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2004; 20(3):231–240. PMID: 15703647.6. Lodato F, Larocca A, D’Errico A, Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug-related liver injury. J Hepatol. 2021; 75(5):1254–1256. PMID: 34256064.7. Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J Hepatol. 2021; 75(3):728–729. PMID: 34116081.8. Avci E, Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: new-onset or flare-up? J Autoimmun. 2021; 125:102745. PMID: 34781161.9. Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, et al. COVID-19 and autoimmunity. Autoimmun Rev. 2020; 19(8):102597. PMID: 32535093.10. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020; 217:108480. PMID: 32461193.11. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021; 21(8):475–484. PMID: 34211186.12. Malonis RJ, Lai JR, Vergnolle O. Peptide-based vaccines: current progress and future challenges. Chem Rev. 2020; 120(6):3210–3229. PMID: 31804810.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Discrimination between Benign and Malignant Post-SARS-CoV-2 Vaccination Lymphadenopathy is Feasible
- Guillain-Barre Syndrome Following SARS-CoV-2 Vaccination: A Case Report
- New-Onset Fulminant Type 1 Diabetes Following SARS-CoV-2 Protein Subunit Vaccine: A Case Report and Literature Review
- Alopecia areata after COVID-19 vaccination
- SARS-CoV-2-Specific T Cell Responses in Patients with COVID-19 and Unexposed Individuals