Kosin Med J.
2022 Mar;37(1):68-74. 10.7180/kmj.22.008.
Hepatic steatosis changes after early gastric cancer surgery
- Affiliations
-
- 1Department of Surgery, Kosin University College of Medicine, Busan, Korea
- 2Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
- KMID: 2527697
- DOI: http://doi.org/10.7180/kmj.22.008
Abstract
- Background
Nonalcoholic fatty liver disease dramatically improves after bariatric surgery, primarily due to improvements in hepatic insulin sensitivity. Since the procedure for gastric cancer surgery is very similar to that for bariatric surgery, we investigated changes in fatty liver following gastrectomy for gastric cancer according to the type of surgery.
Methods
We evaluated hepatic steatosis in 212 early gastric cancer patients using Hounsfield units (HUs) on non-contrast computed tomography preoperatively and 6, 12, and 24 months after surgery. We compared the preoperative and postoperative liver-to-spleen HU ratio according to the type of surgery: Billroth I, Billroth II, and total gastrectomy with Roux-en-Y reconstruction.
Results
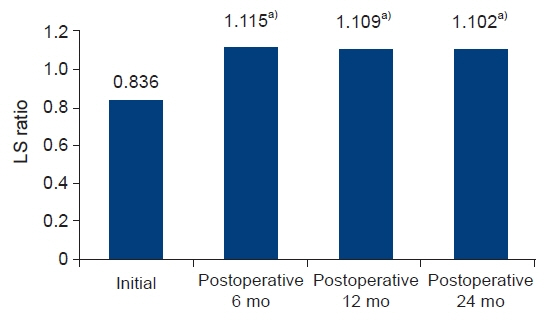
The initial results (liver/spleen HUs and the liver-to-spleen HU ratio) did not significantly differ according to surgical group. After surgery, only patients who underwent total gastrectomy with Roux-en-Y exhibited significant changes in the liver-to-spleen HU ratio at 6 months. In 26 patients who had higher initial HU levels of the spleen than the liver, the liver-to-spleen HU ratio significantly increased from 0.836 to 1.115 at 6 months, 1.109 at 12 months, and 1.102 at 24 months (P<0.01).
Conclusion
Significant changes in hepatic steatosis were found in even normal patients (with higher liver than spleen HU values) who underwent total gastrectomy with Roux-en-Y. Patients who initially had fatty liver also showed a significant increase in the liver-to-spleen HU ratio. These results suggest that total gastrectomy with Roux-en-Y reconstruction can have a positive effect on the improvement of hepatic steatosis.
Keyword
Figure
Reference
-
References
1. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009; 122:248–56.
Article2. Padwal R, Klarenbach S, Wiebe N, Birch D, Karmali S, Manns B, et al. Bariatric surgery: a systematic review and network meta-analysis of randomized trials. Obes Rev. 2011; 12:602–21.
Article3. Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008; 6:1396–402.
Article4. Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009; 137:532–40.
Article5. Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006; 40 Suppl 1:S11–6.6. Park SH. Current status of liver disease in Korea: nonalcoholic fatty liver disease. Korean J Hepatol. 2009; 15 Suppl 6:S34–9.
Article7. Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Serup AK, Albers PH, et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014; 63:1725–37.
Article8. Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee DH, et al. Prediction of cancer incidence and mortality in Korea, 2015. Cancer Res Treat. 2015; 47:142–8.
Article9. Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007; 188:1307–12.
Article10. Jacobs JE, Birnbaum BA, Shapiro MA, Langlotz CP, Slosman F, Rubesin SE, et al. Diagnostic criteria for fatty infiltration of the liver on contrast-enhanced helical CT. AJR Am J Roentgenol. 1998; 171:659–64.
Article11. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–63.12. Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organ Tech Rep Ser. 2000; 894:i–xii. 1–253.13. Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009; 104:861–7.
Article14. Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012; 18:49–54.
Article15. Kang KC, Shin SH, Lee YJ, Heo YS. Influence of gastrectomy for stomach cancer on type 2 diabetes mellitus for patients with a body mass index less than 30 kg/m(2). J Korean Surg Soc. 2012; 82:347–55.
Article16. Clark JM, Alkhuraishi AR, Solga SF, Alli P, Diehl AM, Magnuson TH. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res. 2005; 13:1180–6.
Article17. Angulo P. NAFLD, obesity, and bariatric surgery. Gastroenterology. 2006; 130:1848–52.
Article18. Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006; 101:368–73.
Article19. Dirksen C, Jorgensen NB, Bojsen-Moller KN, Jacobsen SH, Hansen DL, Worm D, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012; 55:1890–901.
Article20. Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010; 51:1584–92.
Article21. Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011; 54:1214–23.
Article22. Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011; 31:1285–97.
Article23. Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, et al. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006; 574(Pt 1):41–53.
Article24. Pournaras DJ, Aasheim ET, Bueter M, Ahmed AR, Welbourn R, Olbers T, et al. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg Obes Relat Dis. 2012; 8:371–4.
Article25. Lindqvist A, Spegel P, Ekelund M, Mulder H, Groop L, Hedenbro J, et al. Effects of ingestion routes on hormonal and metabolic profiles in gastric-bypassed humans. J Clin Endocrinol Metab. 2013; 98:E856–61.
Article26. Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond). 2009; 33:786–95.
Article27. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009; 17:1671–7.
Article28. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening of gastric cancer
- Hepatic Fibrosis and Steatosis in Metabolic Syndrome
- Common Hepatic Artery Originating from Left Gastric Artery: A Rare Variant Encountered in Gastric Cancer Surgery
- Evaluation for prognostic factors following surgical management of gastric cancer patients with hepatic cirrhosis
- Diagnostic Performance of Hepatic Steatosis Algorithms in Korean Population with Metabolic-Associated Fatty Liver Disease