J Pathol Transl Med.
2022 Mar;56(2):81-91. 10.4132/jptm.2021.12.27.
Blocking Toll-like receptor 9 attenuates bleomycin-induced pulmonary injury
- Affiliations
-
- 1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Jouf University, Sakaka, Saudi Arabia
- 2Department of Pathology, Faculty of Veterinary Medicine, Benha University, Tukh, Egypt
- 3Department of Statistics, Faculty of Commerce, Benha University, Benha, Egypt
- 4Department of Animal Medicine (Infectious Diseases), Faculty of Veterinary Medicine, Benha University, Tukh, Egypt
- KMID: 2527192
- DOI: http://doi.org/10.4132/jptm.2021.12.27
Abstract
- Background
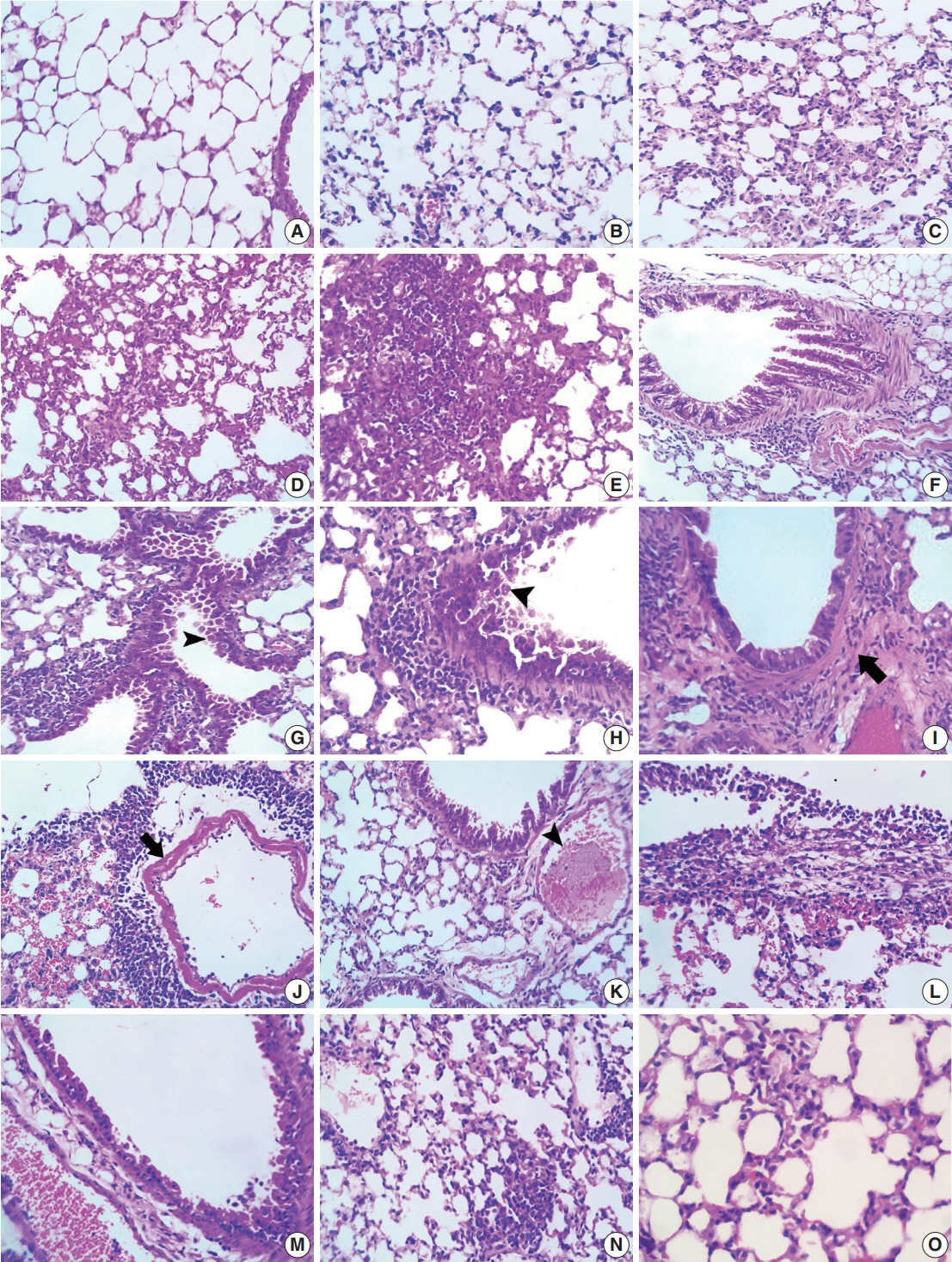
Acute respiratory distress syndrome (ARDS) is one of the most common complications in coronavirus disease 2019 patients suffering from acute lung injury (ALI). In ARDS, marked distortion of pulmonary architecture has been reported. The pulmonary lesions in ARDS include hemodynamic derangements (such as alveolar edema and hemorrhage), vascular and bronchiolar damage, interstitial inflammatory cellular aggregations, and eventually fibrosis. Bleomycin induces ARDS-representative pulmonary damage in mice and rats; therefore, we used bleomycin model mice in our study. Recently, Toll-like receptor 9 (TLR9) was implicated in the development of ARDS and ALI.
Methods
In this study, we evaluated the efficiency of a TLR9 blocker (ODN2088) on bleomycin-induced pulmonary damage. We measured the apoptosis rate, inflammatory reaction, and fibroplasia in bleomycin- and bleomycin + ODN2088-treated mice.
Results
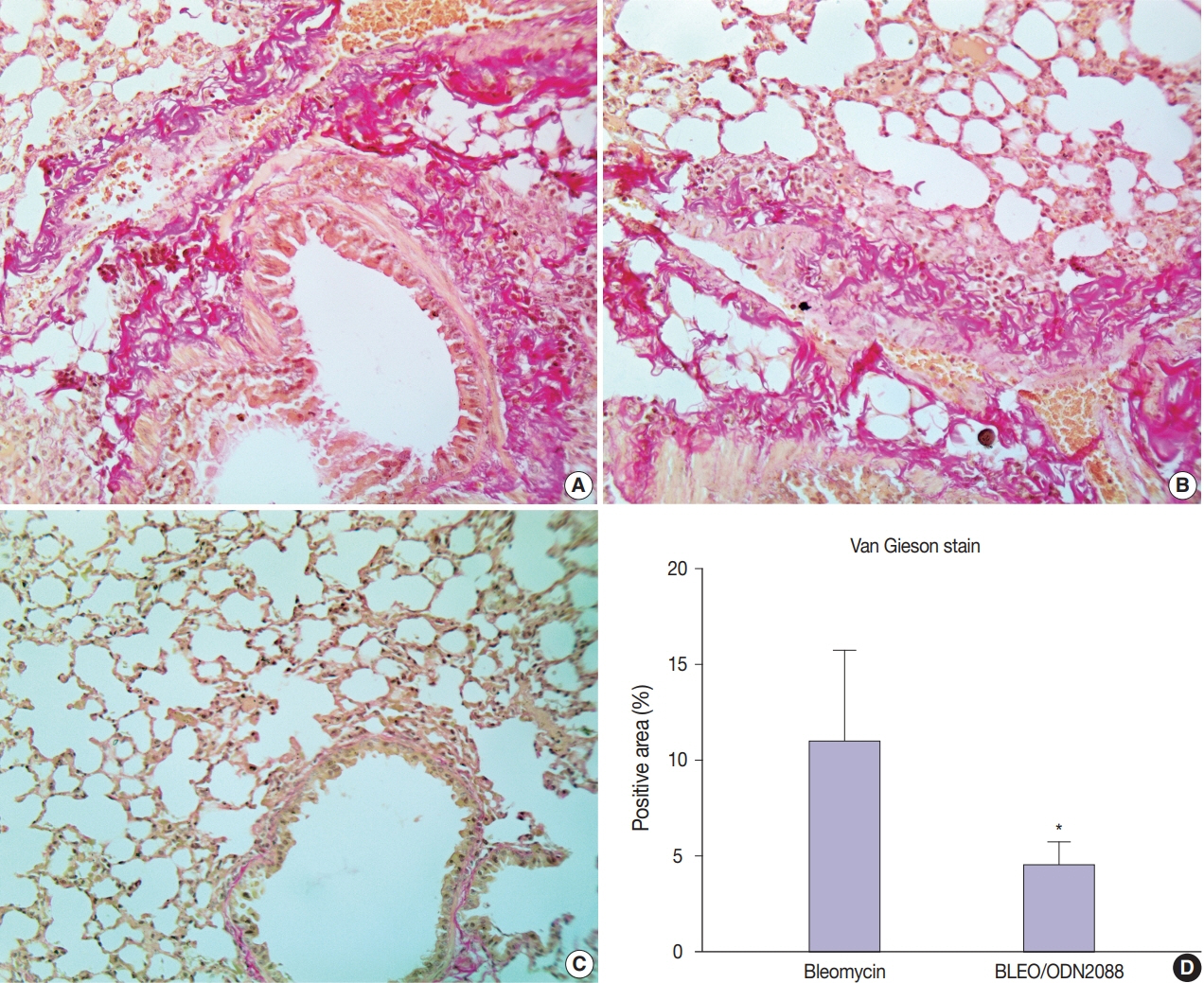
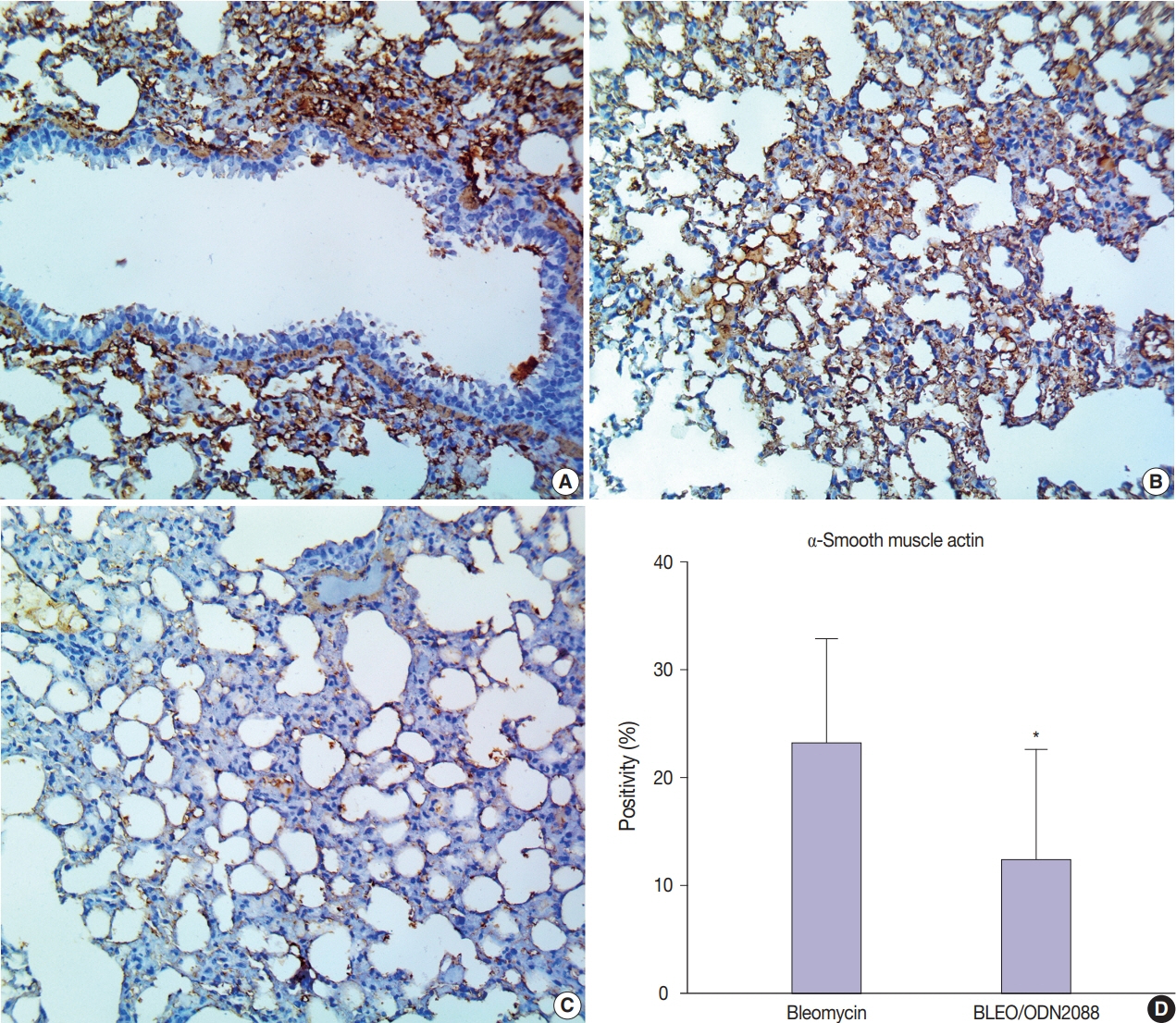
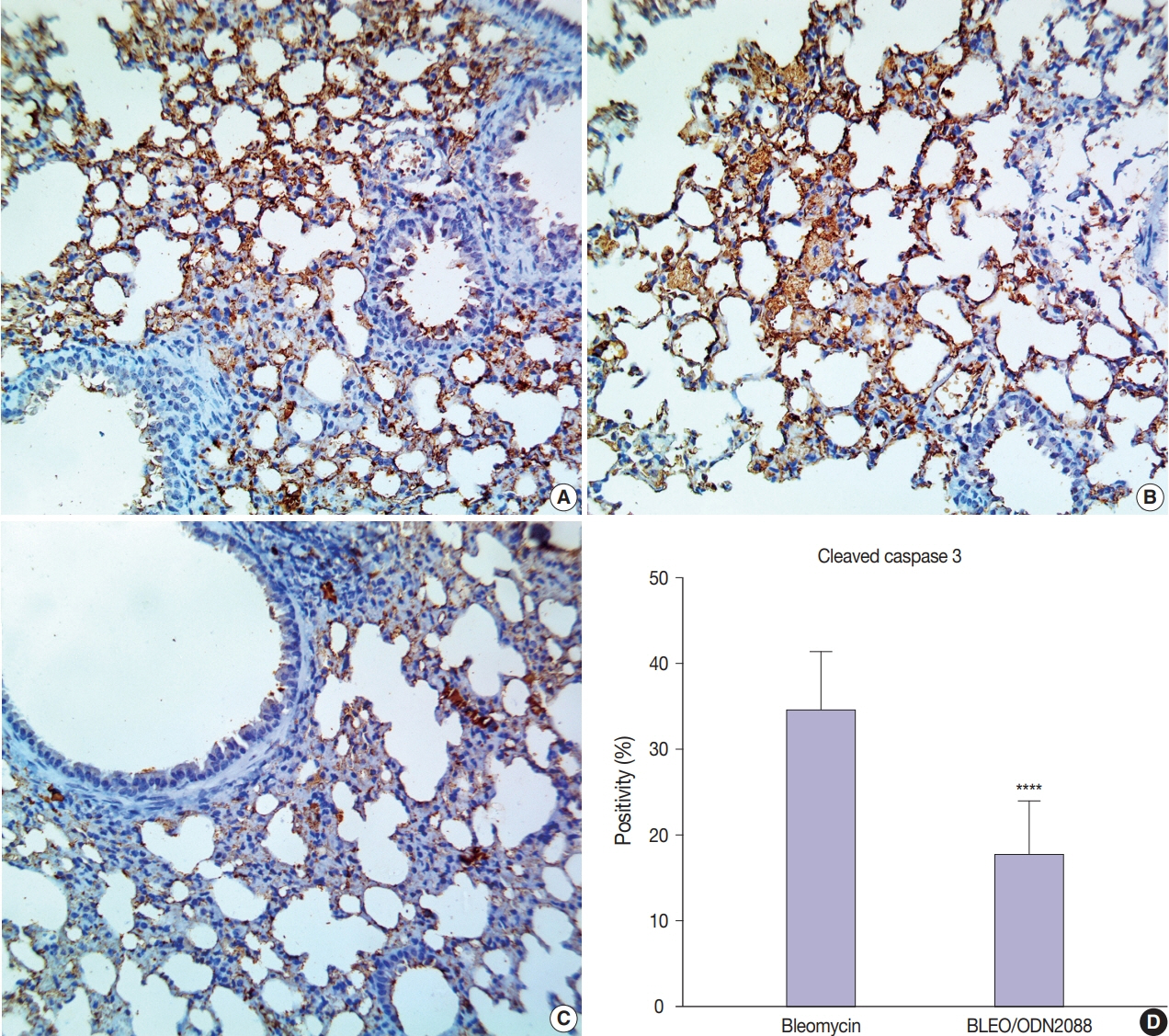
Our results showed a significant amelioration in bleomycin-induced damage to pulmonary architecture following ODN2088 treatment. A marked decrease in pulmonary epithelial and endothelial apoptosis rate as measured by cleaved caspase-3 expression, inflammatory reaction as indicated by tumor necrosis factor α expression, and pulmonary fibrosis as demonstrated by Van Gieson staining and α-smooth muscle actin immunohistochemistry were observed following ODN2088 treatment.
Conclusions
All these findings indicate that blocking downstream TLR9 signaling could be beneficial in prevention or mitigation of ARDS through hemodynamic derangements, inflammation, apoptosis, and fibrosis.
Keyword
Figure
Reference
-
References
1. She J, Jiang J, Ye L, Hu L, Bai C, Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Transl Med. 2020; 9:19.
Article2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–42.
Article3. Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011; 6:147–63.
Article4. Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008; 294:L152–60.
Article5. Moroni M, Giannetta L, Gelosa G, et al. Second-line chemotherapy with bleomycin, methotrexate, and vinorelbine (BMV) for patients with squamous cell carcinoma of the head, neck and esophagus (SCC-HN&E) pretreated with a cisplatin-containing regimen: a phase II study. J Chemother. 2003; 15:394–9.
Article6. Canellos GP. Lymphoma: present and future challenges. Semin Hematol. 2004; 41(4 Suppl 7):26–31.
Article7. Rimmer Y, Chester J, Joffe J, et al. Accelerated BEP: a phase I trial of dose-dense BEP for intermediate and poor prognosis metastatic germ cell tumour. Br J Cancer. 2011; 105:766–72.
Article8. Gasse P, Riteau N, Charron S, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009; 179:903–13.
Article9. Ruscitti F, Ravanetti F, Essers J, et al. Longitudinal assessment of bleomycin-induced lung fibrosis by Micro-CT correlates with histological evaluation in mice. Multidiscip Respir Med. 2017; 12:8.
Article10. Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 2013; 49:167–79.
Article11. Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008; 133:235–49.
Article12. Lafferty EI, Qureshi ST, Schnare M. The role of toll-like receptors in acute and chronic lung inflammation. J Inflamm (Lond). 2010; 7:57.
Article13. Arancibia SA, Beltran CJ, Aguirre IM, et al. Toll-like receptors are key participants in innate immune responses. Biol Res. 2007; 40:97–112.
Article14. Gao W, Xiong Y, Li Q, Yang H. Inhibition of Toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front Physiol. 2017; 8:508.
Article15. Knuefermann P, Baumgarten G, Koch A, et al. CpG oligonucleotide activates Toll-like receptor 9 and causes lung inflammation in vivo. Respir Res. 2007; 8:72.
Article16. Tasaka S, Kamata H, Miyamoto K, et al. Intratracheal synthetic CpG oligodeoxynucleotide causes acute lung injury with systemic inflammatory response. Respir Res. 2009; 10:84.
Article17. Huang L, Chang W, Huang Y, Xu X, Yang Y, Qiu H. Prognostic value of plasma mitochondrial DNA in acute respiratory distress syndrome (ARDS): a single-center observational study. J Thorac Dis. 2020; 12:1320–8.
Article18. Faust HE, Reilly JP, Anderson BJ, et al. Plasma mitochondrial DNA levels are associated with ARDS in trauma and sepsis patients. Chest. 2020; 157:67–76.
Article19. Koupenova M, Mick E, Mikhalev E, Benjamin EJ, Tanriverdi K, Freedman JE. Sex differences in platelet toll-like receptors and their association with cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2015; 35:1030–7.
Article20. El Kebir D, Damlaj A, Makhezer N, Filep JG. Toll-like receptor 9 signaling regulates tissue factor and tissue factor pathway inhibitor expression in human endothelial cells and coagulation in mice. Crit Care Med. 2015; 43:e179–89.
Article21. Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000; 408:740–5.
Article22. Jurk M, Vollmer J. Therapeutic applications of synthetic CpG oligodeoxynucleotides as TLR9 agonists for immune modulation. BioDrugs. 2007; 21:387–401.
Article23. Alzahrani B. Hepatoprotective impact of TLR9 antagonist ODN 2088 against carbon tetrachloride (CCl4)-induced hepatic injury. Am J Biochem Biotechnol. 2020; 16:9–14.
Article24. Akishima Y, Akasaka Y, Ishikawa Y, et al. Role of macrophage and smooth muscle cell apoptosis in association with oxidized low-density lipoprotein in the atherosclerotic development. Mod Pathol. 2005; 18:365–73.
Article25. Wang J, Wang BJ, Yang JC, et al. Research advances in the mechanism of pulmonary fibrosis induced by coronavirus disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang Za Zhi. 2020; 36:691–7.26. Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009; 179:588–94.
Article27. Liu T, De Los Santos FG, Phan SH. The bleomycin model of pulmonary fibrosis. Methods Mol Biol. 2017; 1627:27–42.
Article28. Amin M, Wu V, Odish M, et al. A case of severe ARDS due to bleomycin-induced lung inury requiring ECMO support. Chest. 2020; 158(4 Suppl):A1126–7.29. Daphale A, Acharya S, Shukla S, Alegaonkar S. Bleomycin induced acute respiratory distress syndrome. J Case Rep. 2017; 7:55–7.
Article30. Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009; 2:103–21.
Article31. Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012; 122:2756–62.
Article32. Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008; 40:362–82.
Article33. Alzahrani B, A AMA, Tantawy A. Therapeutic impact of ODN2088 to block TLR9 activity in induced liver fibrosis mice. Pak J Biol Sci. 2021; 24:122–31.
Article34. Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med. 2006; 173:769–76.
Article35. Chandler DB. Possible mechanisms of bleomycin-induced fibrosis. Clin Chest Med. 1990; 11:21–30.
Article36. Reinert T, da Rocha Baldotto CS, Nunes FA, de Souza Scheliga AA. Bleomycin-induced lung injury. J Cancer Res. 2013; 2013:480608.
Article37. Williamson JD, Sadofsky LR, Hart SP. The pathogenesis of bleomycin-induced lung injury in animals and its applicability to human idiopathic pulmonary fibrosis. Exp Lung Res. 2015; 41:57–73.
Article38. Khanmohammadi S, Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021; 93:2735–9.
Article39. Lin L, Luo S, Qin R, et al. Long-term infection of SARS-CoV-2 changed the body’s immune status. Clin Immunol. 2020; 218:108524.
Article40. He L, Ding Y, Zhang Q, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006; 210:288–97.41. Ellson CD, Dunmore R, Hogaboam CM, Sleeman MA, Murray LA. Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014; 51:163–8.
Article42. Kuwano K, Kunitake R, Maeyama T, et al. Attenuation of bleomycininduced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2001; 280:L316–25.
Article43. Gazdhar A, Fachinger P, van Leer C, et al. Gene transfer of hepatocyte growth factor by electroporation reduces bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L529–36.
Article44. Garcia-Revilla J, Deierborg T, Venero JL, Boza-Serrano A. Hyperinflammation and fibrosis in severe COVID-19 patients: galectin-3, a target molecule to consider. Front Immunol. 2020; 11:2069.
Article45. Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol Res. 2015; 97:122–30.
Article46. Zhao Y, Tian B, Sadygov RG, Zhang Y, Brasier AR. Integrative proteomic analysis reveals reprograming tumor necrosis factor signaling in epithelial mesenchymal transition. J Proteomics. 2016; 148:126–38.
Article47. Altintas N, Erboga M, Aktas C, et al. Protective effect of infliximab, a tumor necrosis factor-alfa inhibitor, on bleomycin-induced lung fibrosis in rats. Inflammation. 2016; 39:65–78.
Article48. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007; 127:526–37.
Article49. Anders HJ, Vielhauer V, Eis V, et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J. 2004; 18:534–6.
Article50. Angeles Montero-Fernandez M, Pardo-Garcia R. Histopathology features of the lung in COVID-19 patients. Diagn Histopathol (Oxf). 2021; 27:123–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deficiency of Sphingosine-1-Phosphate Receptor 2 (S1Pâ‚‚) Attenuates Bleomycin-Induced Pulmonary Fibrosis
- Abnormal B Lymphocyte Activation and Function in Systemic Sclerosis
- Nucleic Acid Recognition and Signaling by Toll-like Receptor 9: Compartment-dependent Regulation
- Amelioration of Bleomycin-induced Pulmonary Fibrosis of Rats by an Aldose Reductase Inhibitor, Epalrestat
- A Cse of Severe Bleomycin-Induved Pneumonitis at Non-Hodgkin's Lymphoma