Lab Med Online.
2021 Jan;11(1):55-59. 10.47429/lmo.2021.11.1.55.
Evaluation of the Analytical Performance of the Norudia GA Glycoalbumin Test
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2525781
- DOI: http://doi.org/10.47429/lmo.2021.11.1.55
Abstract
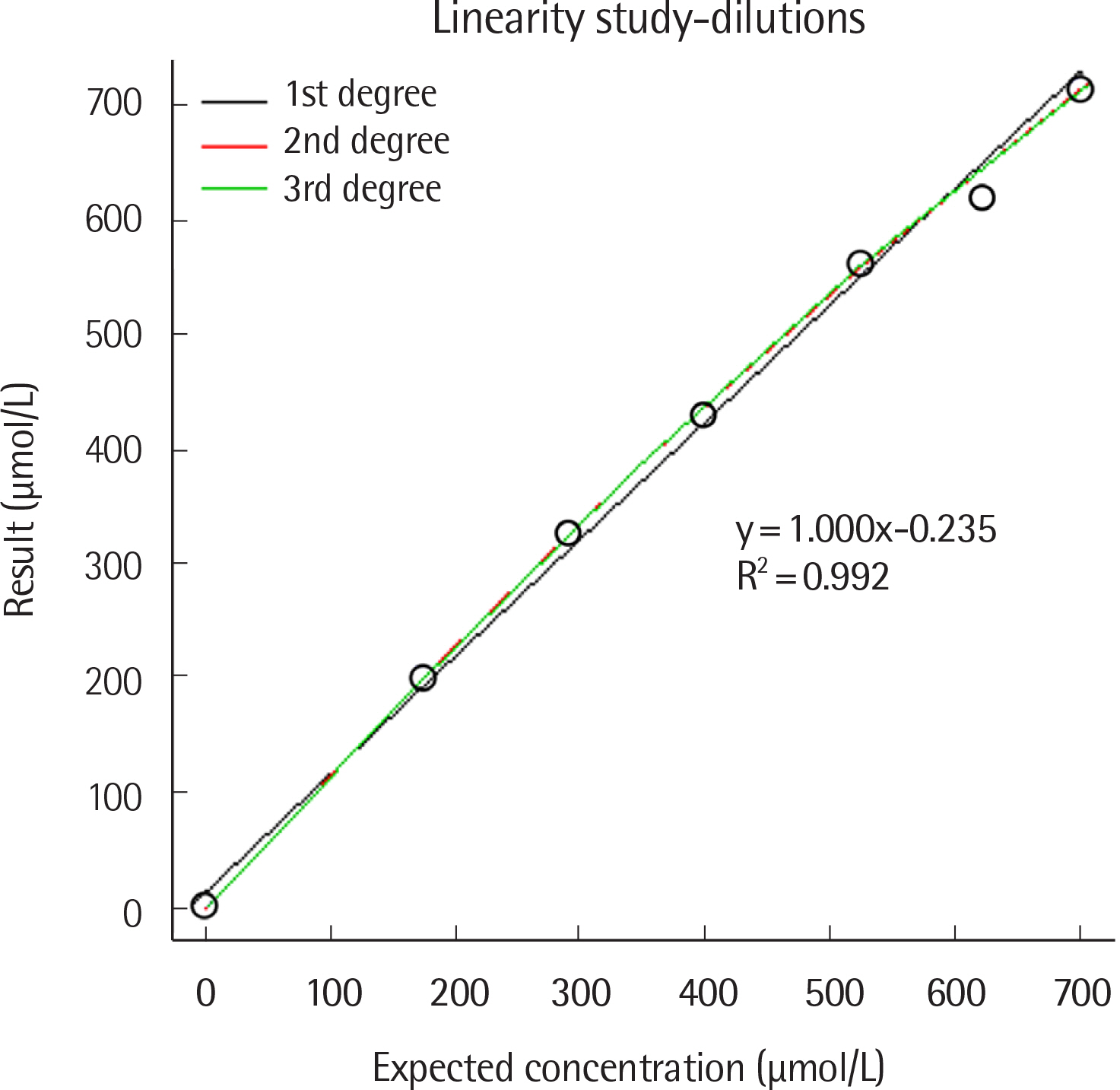
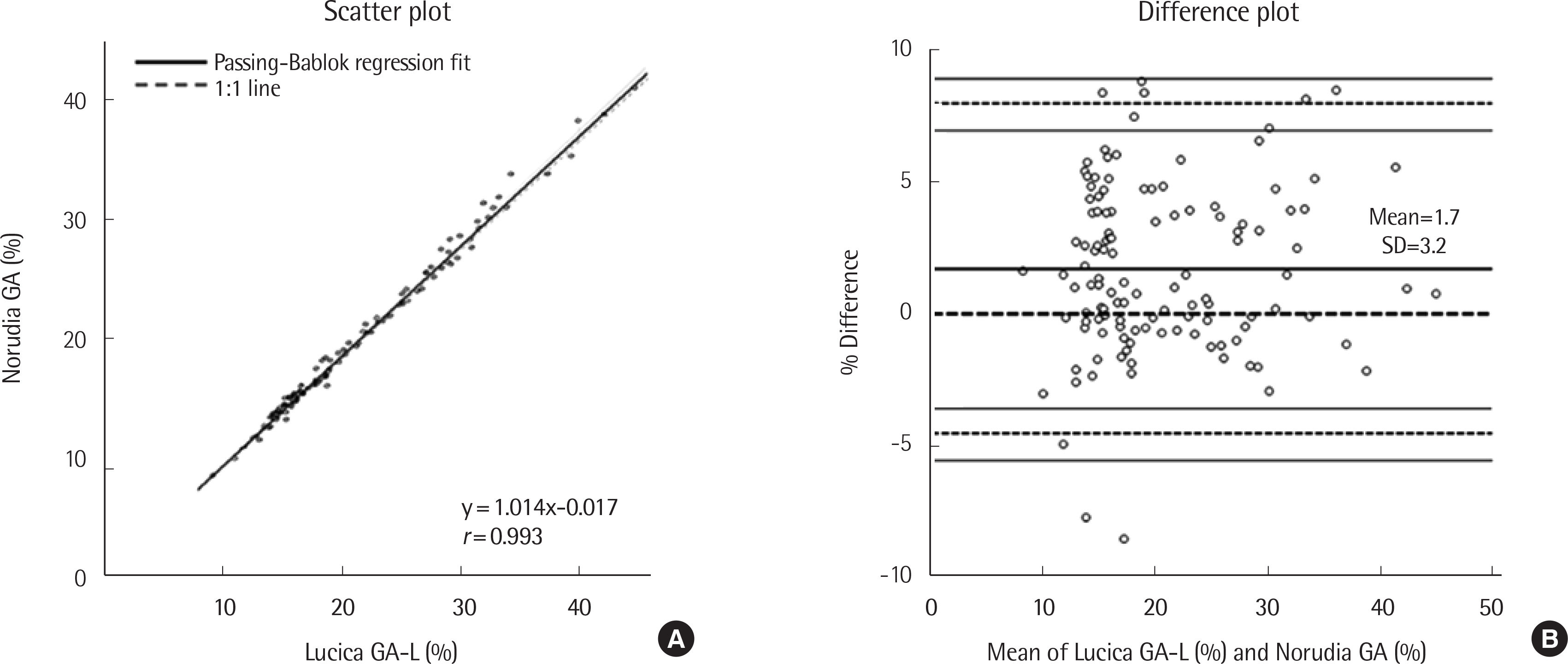
- Blood biomarkers for the diagnosis and monitoring of diabetes mellitus include fasting plasma glucose, HbA1c, glycoalbumin (GA), fructosamine, and 1,5-anhydro-D-glucitol levels. GA has a half-life of approximately three weeks, and its levels are useful for assessing short-term changes in glycemic control. Herein, we evaluated the performance of the Norudia GA assay kit (Sekisui Medical, Japan) using a Roche Modular E170 analyzer (Roche Diagnostics, Germany). We evaluated the precision and linearity of the assay in accordance with the Clinical and Laboratory Standards Institute (CLSI) EP05-A3 and EP06-A guidelines, respectively. A comparison of the Norudia GA and Lucica GA-L (Asahi Kasei Pharma Corporation, Japan) methods was performed by Passing-Bablok regression, using blood samples obtained from 121 patients, in accordance with the CLSI guideline EP09-A3. The reference interval for GA concentration was established based on 120 healthy individuals, according to CLSI guideline EP28-A3C. Repeatability (% CV) ranged from 0.9-2.5% and within-laboratory precision was 2.6-4.5% at three levels. Linearity was observed for GA concentration in the performance ranges used (21.4-713.0 μmol/L). In the equation for the correlation between Norudia GA and Lucica GA-L assay results, the slope and y-intercept were 1.014 (95% confidence interval: 1.000-1.045) and -0.017 (95% confidence interval: -0.444- 0.381), respectively. The reference interval was calculated to be 11.2-17.5%.
Keyword
Figure
Cited by 1 articles
-
음성 간섭을 감소시키도록 개선된 Norudia 당화알부민 시약의 성능평가
Beomki Lee, Sang-Mi Kim, Hyung-Doo Park
Lab Med Online. 2024;14(2):100-105. doi: 10.47429/lmo.2024.14.2.100.
Reference
-
1. American Diabetes Association. 2019; Standards of medical care in diabetes-2019 Abridged for primary care providers. Clin Diabetes. 37:11–34. DOI: 10.2337/cd18-0105. PMID: 30705493. PMCID: PMC6336119.2. Gonen B, Rubenstein A, Rochman H, Tanega SP, Horwitz DL. 1977; Haemoglobin A1: an indicator of the metabolic control of diabetic patients. Lancet. 2:734–7. DOI: 10.1016/S0140-6736(77)90237-9.3. Park HI, Kim YS, Lee J, Kim Y, Shin SJ. 2009; Performance characteristics of glycated albumin and its clinical usefulness in diabetic patients on hemodialysis. Korean J Lab Med. 29:406–14. DOI: 10.3343/kjlm.2009.29.5.406. PMID: 19893349.4. Freitas PAC, Ehlert LR, Camargo JL. 2017; Glycated albumin: a potential biomarker in diabetes. Arch Endocrinol Metab. 61:296–304. DOI: 10.1590/2359-3997000000272. PMID: 28699985.5. Kouzuma T, Uemastu Y, Usami T, Imamura S. 2004; Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 346:135–43. DOI: 10.1016/j.cccn.2004.02.019. PMID: 15256314.6. Sato A, Yada S, Hosoba E, Kanno H, Miura H. 2019; Establishment of glycated albumin unit conversion equation from the standardized value (mmol/mol) to the routinely used value (%). Ann Clin Biochem. 56:204–9. DOI: 10.1177/0004563218808325. PMID: 30282464.7. Testa R, Guerra E, Bonfigli AR, Di Gaetano N, Santini G, Ceriotti F. 2016; Analytical performances of an enzymatic assay for the measurement of glycated albumin. J Appl Lab Med. 1:162–71. DOI: 10.1373/jalm.2016.020446.8. Kohzuma T, Koga M. 2010; Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther. 14:49–51. DOI: 10.1007/BF03256353. PMID: 20121290.9. Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. 2011; Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol. 5:1455–62. DOI: 10.1177/193229681100500619. PMID: 22226265. PMCID: PMC3262714.10. Paroni R, Ceriotti F, Galanello R, Battista Leoni G, Panico A, Scurati E, et al. 2007; Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem. 40:1398–405. DOI: 10.1016/j.clinbiochem.2007.08.001. PMID: 17919531.11. Clinical and Laboratory Standards Institute. 2014. Evaluation of precision of quantitative measurement procedures; Approved guideline-Third edition. EP05-A3. Clinical and Laboratory Standards Institute;Wayne, PA:12. Clinical and Laboratory Standards Institute. 2003. Evaluation of the linearity of quantitative measurement procedures; a statistical approach; Approved guideline. EP06-A. Clinical and Laboratory Standards Institute;Wayne, PA:13. Clinical and Laboratory Standards Institute. 2013. Measurement procedure comparison and bias estimation using patient samples; Approved guideline-Third edition. CLSI document EP09-A3. Clinical and Laboratory Standards Institute;Wayne, PA:14. Clinical and Laboratory Standards Institute. 2010. Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guideline-Third edition. EP28-A3C. Clinical and Laboratory Standards Institute;Wayne, PA:15. Reed AH, Henry RJ, Mason WB. 1971; Influence of statistical method used on the resulting estimate of normal range. Clin Chem. 17:275–84. DOI: 10.1093/clinchem/17.4.275. PMID: 5552364.16. Danese E, Montagnana M, Nouvenne A, Lippi G. 2015; Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 9:169–76. DOI: 10.1177/1932296814567227. PMID: 25591856. PMCID: PMC4604592.17. Huh JH, Kim KJ, Lee BW, Kim DW, Kang ES, Cha BS, et al. 2014; The relationship between BMI and glycated albumin to glycated hemoglobin (GA/A1c) ratio according to glucose tolerance status. PLoS One. 9:e89478. DOI: 10.1371/journal.pone.0089478. PMID: 24586809. PMCID: PMC3938490.18. Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. 2007; Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 378:48–52. DOI: 10.1016/j.cca.2006.10.013. PMID: 17141207.19. Westgard QC. Desirable Biological Variation Database specifications. https://www.westgard.com/biodatabase1.htm. Last updated in 2014.20. Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, et al. 2011; Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 54:3028–36. DOI: 10.1007/s00125-011-2310-6. PMID: 21947435.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the ARKRAY ADAMS Bridge System Comprising Glucose GA-1171 and HbA1c HA-8180 Analyzers
- Evaluation of Analytical Performance of an Automated Glycated Hemoglobin Analyzer, HLC-723 G11
- The LEAP Checklist for Laboratory Evaluation and Analytical Performance Characteristics Reporting of Clinical Measurement Procedures
- Analytical Performance Evaluation of the ALFIS Cardiac Troponin I Assay
- Performance Evaluation of CareSens PRO Glucose Monitoring System