Lab Med Online.
2021 Jan;11(1):40-46. 10.47429/lmo.2021.11.1.40.
Next-Generation Sequencing Myeloid Panel Test for Patients with AML and MDS: Experience in a Tertiary Care Hospital
- Affiliations
-
- 1Department of Laboratory Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- KMID: 2525779
- DOI: http://doi.org/10.47429/lmo.2021.11.1.40
Abstract
- Background
Next-generation sequencing (NGS) technology can be used for detecting gene mutations in various patients. The NGS myeloid panel test can be specifically used for patients with a myeloid neoplasm. In this study, the NGS myeloid panel test was used for patients with AML and MDS, and the results are summarized and reported retrospectively.
Methods
Thirty-two NGS myeloid panel test results were reviewed retrospectively. Oncomine Myeloid Research Assay (Thermo Fisher Scientific, USA) and Ion Torrent S5 XL (Thermo Fisher Scientific) were used for sequencing, and variant annotation was performed using Ion Reporter Software (Thermo Fisher Scientific). Filtered variants were classified into tiers 1, 2, and 3, according to the Association for Molecular Pathology guidelines.
Results
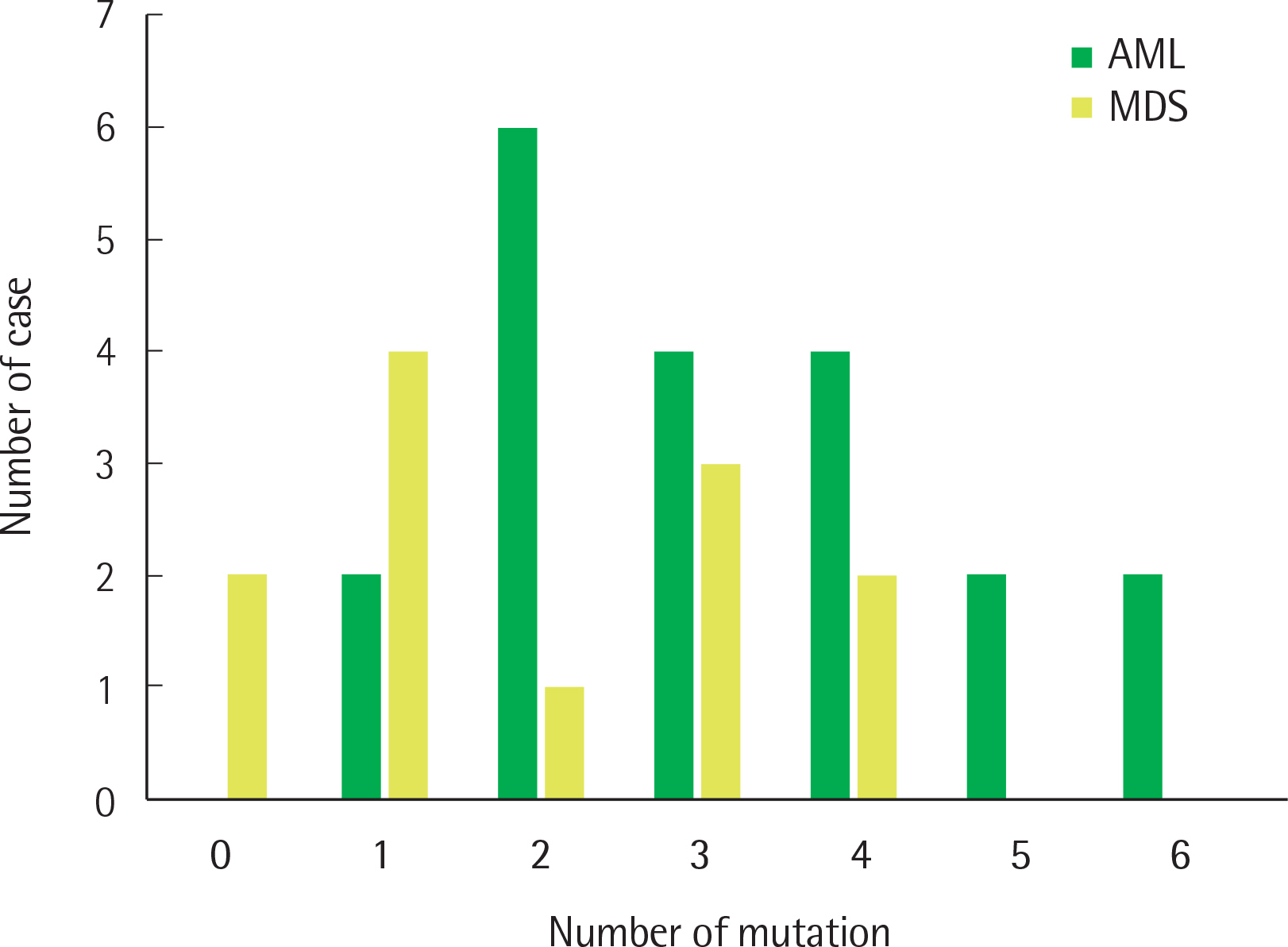
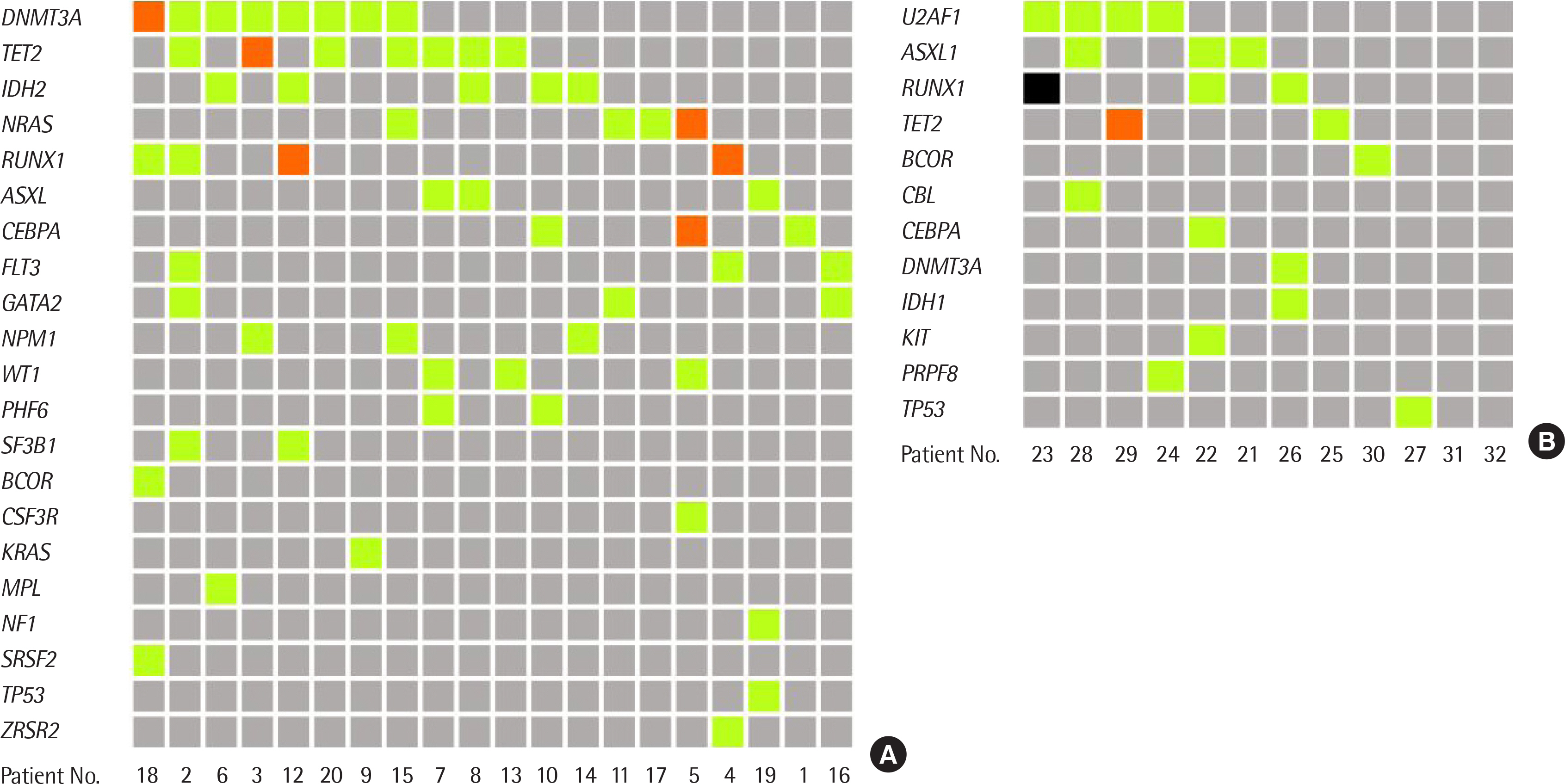
Eighty-seven variants were detected from 32 cases, and 30 cases (94%) had at least 1 variant classified into tier 1 or 2. The most frequently detected mutated gene in patients with AML was DNMT3A (N = 8) and that in patients with MDS was U2AF1 (N = 4). Gene mutations were not detected in patients with MDS with single lineage dysplasia.
Conclusions
Clinically useful genetic mutations were found in patients with AML and MDS through an NGS myeloid panel test. Although there are limitations to this study due to the small number of cases, some differences were found between the results of this study and the genetic profiles of AML patients in other studies. Further evaluation of the genetic profile of myeloid neoplasm is needed, and the NGS myeloid panel test can be useful for this.
Keyword
Figure
Reference
-
1. Duncavage EJ, Tandon B. 2015; The utility of next-generation sequencing in diagnosis and monitoring of acute myeloid leukemia and myelodysplastic syndromes. Int J Lab Hematol. 37:115–21. DOI: 10.1111/ijlh.12361. PMID: 25976969.2. Shumilov E, Flach J, Kohlmann A, Banz Y, Bonadies N, Fiedler M, et al. 2018; Current status and trends in the diagnostics of AML and MDS. Blood Rev. 32:508–19. DOI: 10.1016/j.blre.2018.04.008. PMID: 29728319.3. Alonso CM, Llop M, Sargas C, Pedrola L, Panadero J, Hervás D, et al. 2019; Clinical utility of a next-generation sequencing panel for acute myeloid leukemia diagnostics. J Mol Diagn. 21:228–40. DOI: 10.1016/j.jmoldx.2018.09.009. PMID: 30576870.4. Luthra R, Chen H, Roy-Chowdhuri S, Singh RR. 2015; Next-generation sequencing in clinical molecular diagnostics of cancer: advantages and challenges. Cancers (Basel). 7:2023–36. DOI: 10.3390/cancers7040874. PMID: 26473927. PMCID: PMC4695874.5. Papaemmanuil E, Gerstung M, Bullinger L1, Gaidzik VI1, Paschka P, Roberts ND, et al. 2016; Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 374:2209–21. DOI: 10.1056/NEJMoa1516192. PMID: 27276561. PMCID: PMC4979995.6. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. 2013; Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 368:2059–74. DOI: 10.1056/NEJMoa1301689. PMID: 23634996. PMCID: PMC3767041.7. Docking TR, Karsan A. 2019; Genomic testing in myeloid malignancy. Int J Lab Hematol. 41(S):117–25. DOI: 10.1111/ijlh.13022. PMID: 31069982.8. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. 2016; The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127:2391–405. DOI: 10.1182/blood-2016-03-643544. PMID: 27069254.9. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. 2017; Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 129:424–47. DOI: 10.1182/blood-2016-08-733196. PMID: 27895058. PMCID: PMC5291965.10. Stein EM. 2015; Moleculary targeted therapies for acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2015:579–83. DOI: 10.1182/asheducation-2015.1.579. PMID: 26637775.11. Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. 2011; Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N Engl J Med. 365:1384–95. DOI: 10.1056/NEJMoa1103283. PMID: 21995386. PMCID: PMC3322589.12. Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. 2011; Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 364:2496–506. DOI: 10.1056/NEJMoa1013343. PMID: 21714648. PMCID: PMC3159042.13. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. 1997; International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 89:2079–88. DOI: 10.1182/blood.V89.6.2079. PMID: 9058730.14. Kim B, Lee H, Jang J, Kim SJ, Lee ST, Cheong JW, et al. 2019; Targeted next generation sequencing can serve as an alternative to conventional tests in myeloid neoplasm. PLos ONE. 14:e0212228. DOI: 10.1371/journal.pone.0212228. PMID: 30840646. PMCID: PMC6402635.15. Bacher U, Shumilov E, Flach J, Porret N, Joncourt R, Wiedemann G, et al. 2018; Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J. 8:113. DOI: 10.1038/s41408-018-0148-6. PMID: 30420667. PMCID: PMC6232163.16. Thorvaldsdóttir H, Robinson JT, Mesirov JP. 2013; Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 14:178–92. DOI: 10.1093/bib/bbs017. PMID: 22517427. PMCID: PMC3603213.17. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. 2017; Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 19:4–23. DOI: 10.1016/j.jmoldx.2016.10.002. PMID: 27993330. PMCID: PMC5707196.18. Levy MA, Santos S, Kerkhof J, Stuart A, Aref-Eshghi E, Guo F, et al. 2019; Implementation of an NGS-based sequencing and gene fusion panel for clinical screening of patients with suspected hematologic malignancies. Eur J Haematol. 103:178–89. DOI: 10.1111/ejh.13272. PMID: 31177553.19. Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. 2010; DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 363:2424–33. DOI: 10.1056/NEJMoa1005143. PMID: 21067377. PMCID: PMC3201818.20. Shin SY, Lee ST, Kim HJ, Cho EH, Kim JW, Park S, et al. 2016; Mutation profiling of 19 candidate genes in acute myeloid leukemia suggests significance of DNMT3A mutations. Oncotarget. 7:54825–37. DOI: 10.18632/oncotarget.10240. PMID: 27359055. PMCID: PMC5342384.21. Kennedy JA, Ebert BL. 2017; Clinical implications of genetic mutations in myelodysplastic syndrome. J Clin Oncol. 35:968–74. DOI: 10.1200/JCO.2016.71.0806. PMID: 28297619. PMCID: PMC5455680.22. Li B, Zou D, Yang S, Ouyang G, Mu Q. 2019; Prognostic significance of U2AF1 mutations in myelodysplastic syndromes: a meta-analysis. J Int Med Res. 11:300060519891013. DOI: 10.1177/0300060519891013. PMID: 31826693.23. Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. 2013; Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 122:3616–27. DOI: 10.1182/blood-2013-08-518886. PMID: 24030381. PMCID: PMC3837510.24. Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. 2014; Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 28:241–7. DOI: 10.1038/leu.2013.336. PMID: 24220272. PMCID: PMC3918868.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myelodysplastic syndrome with genetic predisposition
- A Novel Germline Mutation in DDX41 Predisposed to Myelodysplasia/Acute Myeloid Leukemia
- Development of a Next-generation Sequencing-based Gene Panel Test to Detect Measurable Residual Disease in Acute Myeloid Leukemia
- Mesenchymal stromal cells in myeloid malignancies
- Implications of the 5th Edition of the World Health Organization Classification and International Consensus Classification of Myeloid Neoplasm in Myelodysplastic Syndrome With Excess Blasts and Acute Myeloid Leukemia