Nutr Res Pract.
2022 Feb;16(1):33-45. 10.4162/nrp.2022.16.1.33.
Effects of taurine and ginseng extracts on energy metabolism during exercise and their anti-fatigue properties in mice
- Affiliations
-
- 1Physical Activity & Performance Institute, Konkuk University, Seoul 05029, Korea
- 2Department of Medical Science, School of Medicine, Konkuk University, Seoul 05029, Korea
- 3Research and Development Center, UMUST R&D Corporation, Seoul 05029, Korea
- 4Department of Anatomy, College of Korean Medicine, Semyung University, Jecheon 27136, Korea
- 5Department of Nuclear Medicine, Ewha Womans University Seoul Hospital, Ewha Womans University College of Medicine, Seoul 07804, Korea
- 6Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea
- 7Seoul Center, Korea Basic Science Institute, Seoul 02841, Korea
- 8Department of Sports Healthcare management, Namseoul University, Cheonan 31020, Korea
- KMID: 2525511
- DOI: http://doi.org/10.4162/nrp.2022.16.1.33
Abstract
- BACKGROUND/OBJECTIVES
Ginseng extract (GSE) and taurine (TR) are widely used antifatigue resources in functional foods. However, the mechanism underlying the antifatigue effects of GSE and TR are still unclear. Hence, we investigated whether GSE and TR have synergistic effects against fatigue in mice.
MATERIALS/METHODS
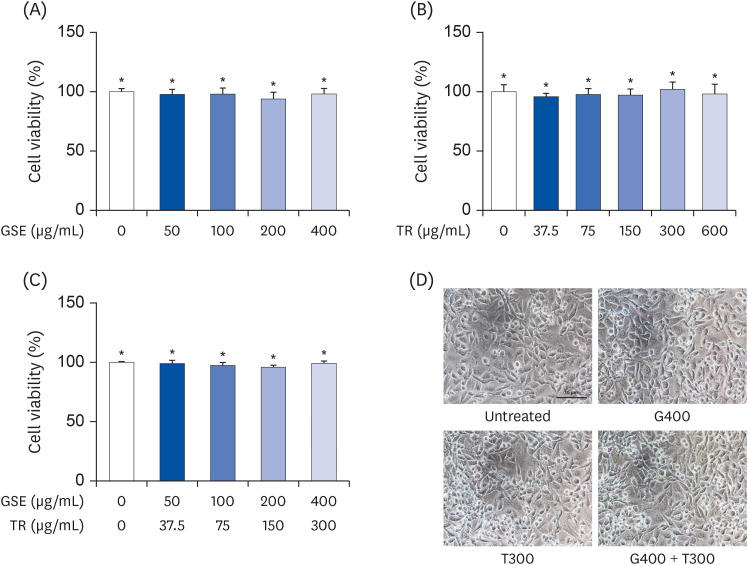
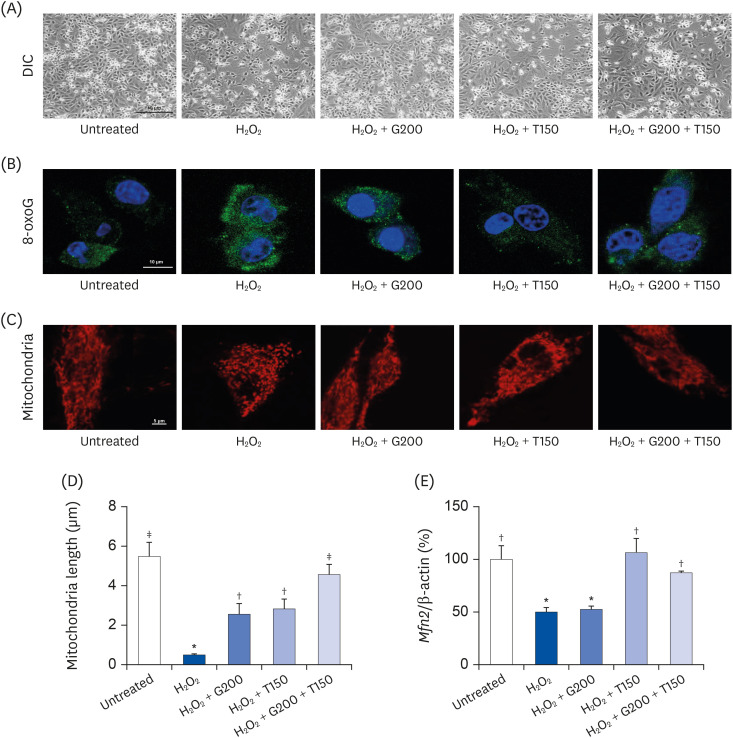
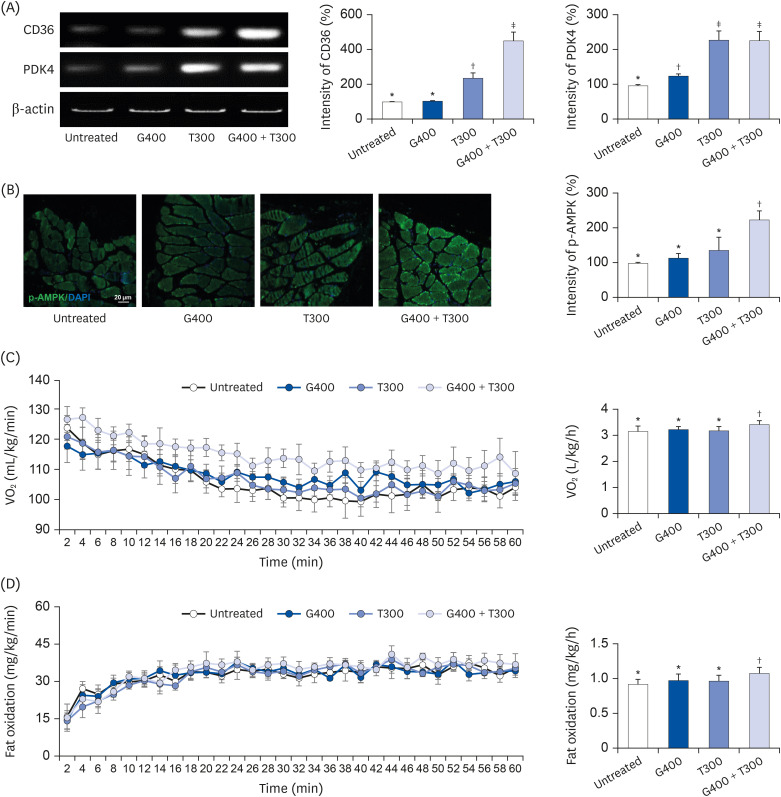
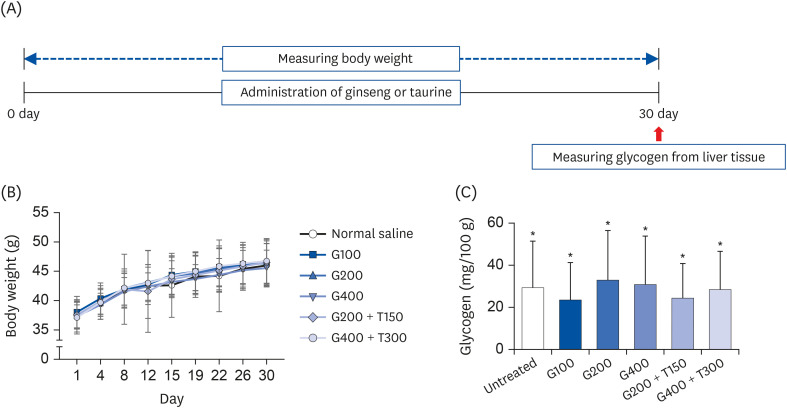
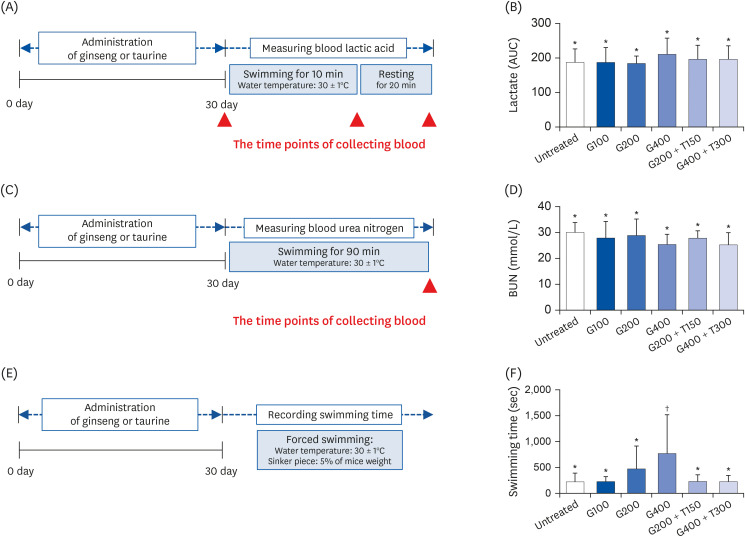
L6 cells were treated with different concentrations of TR and GSE, and cell viability was determined using 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium. Oxidative stress was analyzed by immunocytochemistry using MitoTracker™ Red FM and an anti-8-oxoguanine antibody. Respiratory gas analysis was performed to investigate metabolism. Expression of an activated protein kinase was analyzed using immunohistochemistry. Gene expression of cluster of differentiation 36 and pyruvate dehydrogenase lipoamide kinase isozyme 4 was measured using reverse transcription– polymerase chain reaction. Mice were orally administered TR, GSE, or their combination for 30 days, and then fatigue-related parameters, including lactate, blood urea nitrogen, and glycogen, were measured after forced swimming.
RESULTS
TR and GSE reduced oxidative stress levels in hydrogen peroxide-stimulated L6 cells and enhanced the oxygen uptake and lipid metabolism in mice after acute exercise. After oral administration of TR or GSE for 30 days, the fatigue-related parameters did not change in mice. However, the mice administered GSE (400 mg/kg/day) alone for 30 days could swim longer than those from the other groups. Further, no synergistic effect was observed after the swimming exercise in mice treated with the TR and GSE combination for 30 days.
CONCLUSIONS
Taken together, our data suggest that TR and GSE may exert antifatigue effects in mice after acute exercise by enhancing oxygen uptake and lipid oxidation.
Keyword
Figure
Reference
-
1. Son CG. Review of the prevalence of chronic fatigue worldwide. J Korean Orient Med. 2012; 33:25–33.2. Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020; 18:100. PMID: 32093722.
Article3. Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017; 49:e384. PMID: 28983090.
Article4. Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology (Oxford). 2011; 50:1009–1018. PMID: 21285230.
Article5. Liu J, Du C, Wang Y, Yu Z. Anti-fatigue activities of polysaccharides extracted from Hericium erinaceus . Exp Ther Med. 2015; 9:483–487. PMID: 25574220.6. Aoi W, Naito Y, Yoshikawa T. Exercise and functional foods. Nutr J. 2006; 5:15. PMID: 16749944.
Article7. Chen Y, Michalak M, Agellon LB. Importance of nutrients and nutrient metabolism on human health. Yale J Biol Med. 2018; 91:95–103. PMID: 29955217.8. Batatinha HA, da Costa CE, de França E, Dias IR, Ladeira AP, Rodrigues B, de Lira FS, Correia SC, Caperuto EC. Carbohydrate use and reduction in number of balance beam falls: implications for mental and physical fatigue. J Int Soc Sports Nutr. 2013; 10:32. PMID: 23875791.
Article9. Kim J, Lim K. Relationship between FAT/CD36 protein in skeletal muscle and whole-body fat oxidation in endurance-trained mice. J Exerc Nutrition Biochem. 2016; 20:48–52.
Article10. Schenk S, Horowitz JF. Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am J Physiol Endocrinol Metab. 2006; 291:E254–60. PMID: 16670153.
Article11. Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Neufer PD. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol. 2002; 541:261–271. PMID: 12015434.
Article12. Kamo T, Kurose S, Ohno H, Murata M, Saito T, Kimura Y. Association of epigenetics of the PDK4 gene in skeletal muscle and peripheral blood with exercise therapy following artificial knee arthroplasty. J Physiol Anthropol. 2020; 39:7. PMID: 32216839.
Article13. Rathmacher JA, Fuller JC Jr, Baier SM, Abumrad NN, Angus HF, Sharp RL. Adenosine-5′-triphosphate (ATP) supplementation improves low peak muscle torque and torque fatigue during repeated high intensity exercise sets. J Int Soc Sports Nutr. 2012; 9:48. PMID: 23046855.
Article14. Matsui T, Omuro H, Liu YF, Soya M, Shima T, McEwen BS, Soya H. Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proc Natl Acad Sci U S A. 2017; 114:6358–6363. PMID: 28515312.
Article15. Kim J, Hwang H, Park J, Yun HY, Suh H, Lim K. Silk peptide treatment can improve the exercise performance of mice. J Int Soc Sports Nutr. 2014; 11:35. PMID: 25050085.
Article16. Midzak AS, Chen H, Aon MA, Papadopoulos V, Zirkin BR. ATP synthesis, mitochondrial function, and steroid biosynthesis in rodent primary and tumor Leydig cells. Biol Reprod. 2011; 84:976–985. PMID: 21228212.17. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13. PMID: 19061483.
Article18. Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008; 88:287–332. PMID: 18195089.
Article19. Mach J, Midgley AW, Dank S, Grant RS, Bentley DJ. The effect of antioxidant supplementation on fatigue during exercise: potential role for NAD+(H). Nutrients. 2010; 2:319–329. PMID: 22254024.20. Kim HG, Cho JH, Yoo SR, Lee JS, Han JM, Lee NH, Ahn YC, Son CG. Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013; 8:e61271. PMID: 23613825.
Article21. Schaffer S, Kim HW. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (Seoul). 2018; 26:225–241. PMID: 29631391.
Article22. Ripps H, Shen W. Review: taurine: a "very essential" amino acid. Mol Vis. 2012; 18:2673–2686. PMID: 23170060.23. Kim J, Lee KP, Beak S, Kang HR, Kim YK, Lim K. Effect of black chokeberry on skeletal muscle damage and neuronal cell death. J Exerc Nutrition Biochem. 2019; 23:26–31.
Article24. Kim J, Park J, Kim N, Park HY, Lim K. Inhibition of androgen receptor can decrease fat metabolism by decreasing carnitine palmitoyltransferase I levels in skeletal muscles of trained mice. Nutr Metab (Lond). 2019; 16:82. PMID: 31788014.
Article25. Chung N, Lim K. Influence of high fat and different types of carbohydrate diet on energy metabolism in growing mice. J Exerc Nutrition Biochem. 2019; 23:1–12.
Article26. Kim J, Park J, Kim B, Lee CH, Lim K, Suh H. Effects of silk peptides administration on fat utilization over a whole day in mice. J Exerc Nutrition Biochem. 2016; 20:53–59.
Article27. Liu Y, Liu C. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J Food Drug Anal. 2016; 24:738–745. PMID: 28911611.28. Xu M, Liang R, Li Y, Wang J. Anti-fatigue effects of dietary nucleotides in mice. Food Nutr Res. 2017; 61:1334485. PMID: 28659748.
Article29. Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017; 49:e384. PMID: 28983090.
Article30. Costill DL, Hargreaves M. Carbohydrate nutrition and fatigue. Sports Med. 1992; 13:86–92. PMID: 1561511.31. Hawley JA. The fuels for exercise. Aust J Nutr Diet. 2001; 58:S19–22.32. Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol (1985). 2008; 104:853–860. PMID: 18006866.
Article33. Teng YS, Wu D. Anti-fatigue effect of green tea polyphenols (-)-epigallocatechin-3-gallate (EGCG). Pharmacogn Mag. 2017; 13:326–331. PMID: 28539729.
Article34. Askew EW. Role of fat metabolism in exercise. Clin Sports Med. 1984; 3:605–621. PMID: 6571234.35. Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004; 279:36235–36241. PMID: 15161924.36. Takahashi Y, Tamura Y, Matsunaga Y, Kitaoka Y, Terada S, Hatta H. Effects of taurine administration on carbohydrate metabolism in skeletal muscle during the post-exercise phase. J Nutr Sci Vitaminol (Tokyo). 2016; 62:257–264. PMID: 27725411.
Article37. Song YB, An YR, Kim SJ, Park HW, Jung JW, Kyung JS, Hwang SY, Kim YS. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J Sci Food Agric. 2012; 92:388–396. PMID: 21918993.
Article38. Shi DY, Xie FZ, Zhai C, Stern JS, Liu Y, Liu SL. The role of cellular oxidative stress in regulating glycolysis energy metabolism in hepatoma cells. Mol Cancer. 2009; 8:32. PMID: 19497135.
Article39. Hwang H, Kim J, Lim K. The effect of a 2-week red ginseng supplementation on food efficiency and energy metabolism in mice. Nutrients. 2020; 12:1726.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basic Study on the Effect of Korean Ginseng upon Fracture Healing of the Bone
- Effects of Dietary Supplementation of Taurine, Carnitine or Glutamine on Endurance Exercise Performance and Fatigue Parameters in Athletes
- Beneficial Effects of Taurine on Metabolic Parameters in Animals and Humans
- Anxiolytic Action of Taurine via Intranasal Administration in Mice
- A Key Metabolic Regulator of Bone and Cartilage Health