Clin Endosc.
2022 Jan;55(1):33-40. 10.5946/ce.2021.212.
Contamination Rates in Duodenoscopes Reprocessed Using Enhanced Surveillance and Reprocessing Techniques: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Digestive Disease Institute, Virginia Mason Medical Center, Seattle, WA, USA
- 2Indiana University Health, Indianapolis, IN, USA
- 3University of Nevada, Las Vegas, NV, USA
- 4Mayo Clinic, Rochester, MN, USA
- KMID: 2525045
- DOI: http://doi.org/10.5946/ce.2021.212
Abstract
- Background/Aims
Multiple outbreaks of multidrug-resistant organisms have been reported worldwide due to contaminated duodenoscopes. In 2015, the United States Food and Drug Administration recommended the following supplemental enhanced surveillance and reprocessing techniques (ESRT) to improve duodenoscope disinfection: 1. microbiological culture, 2. ethylene oxide sterilization, 3. liquid chemical sterilant processing system, and 4. double high-level disinfection. A systematic review and metaanalysis was performed to assess the impact of ESRT on the contamination rates.
Methods
A thorough and systematic search was performed across several databases and conference proceedings from inception until January 2021, and all studies reporting the effectiveness of various ESRTs were identified. The pooled contamination rates of post-ESRT duodenoscopes were estimated using the random effects model.
Results
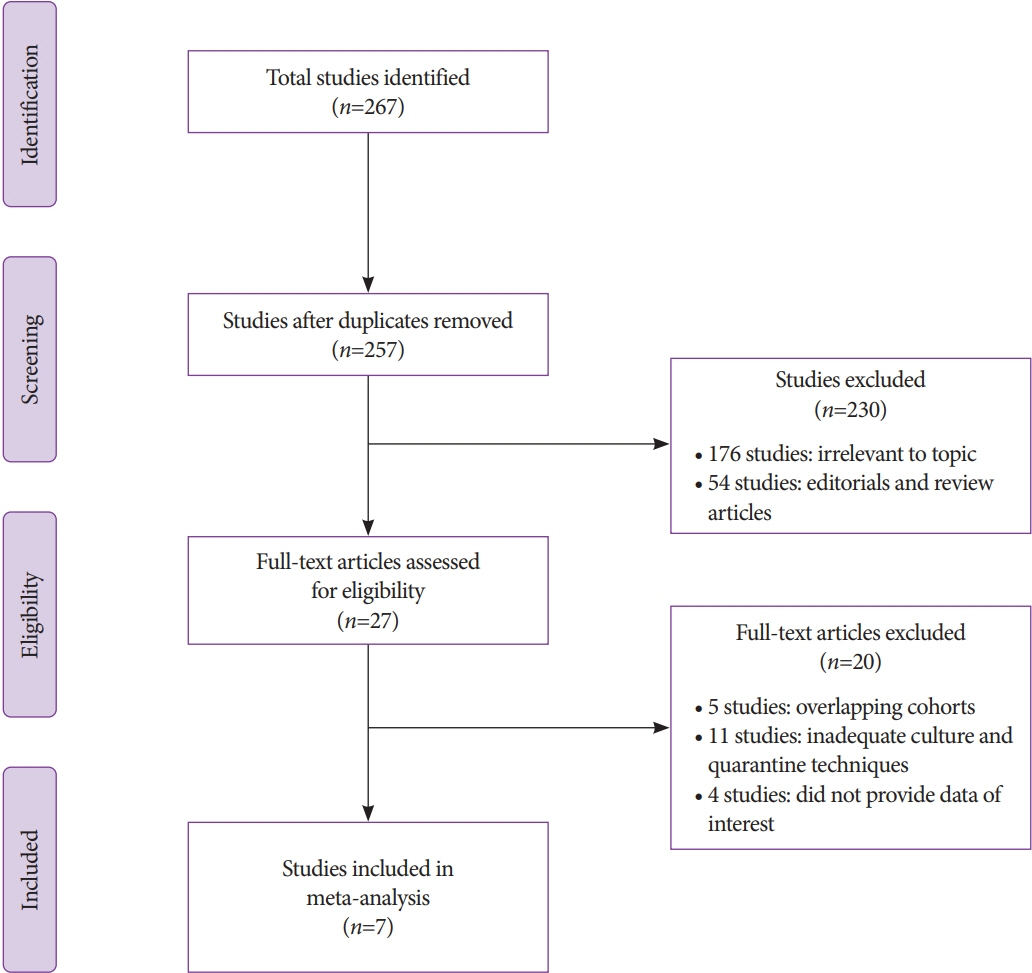
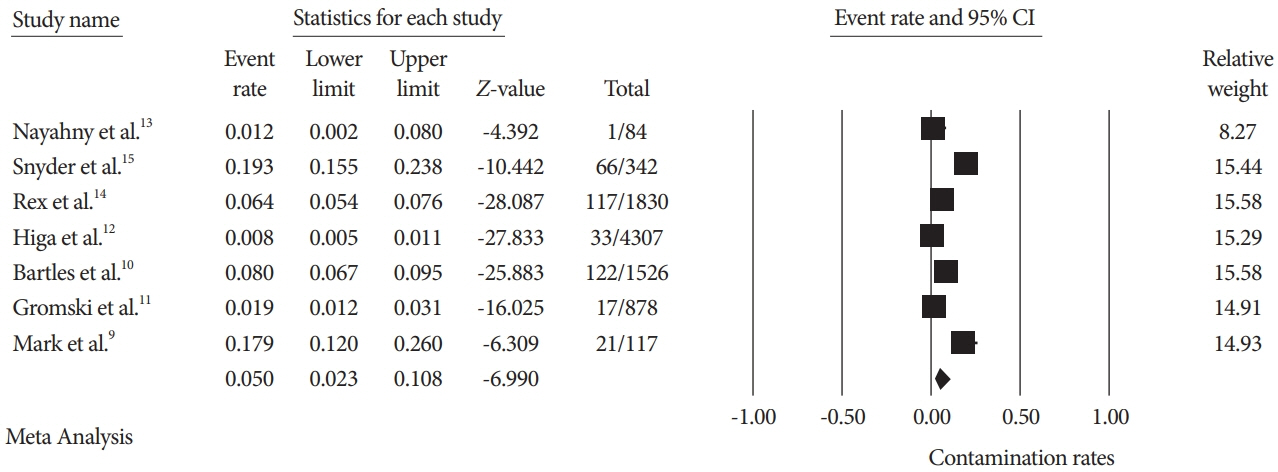
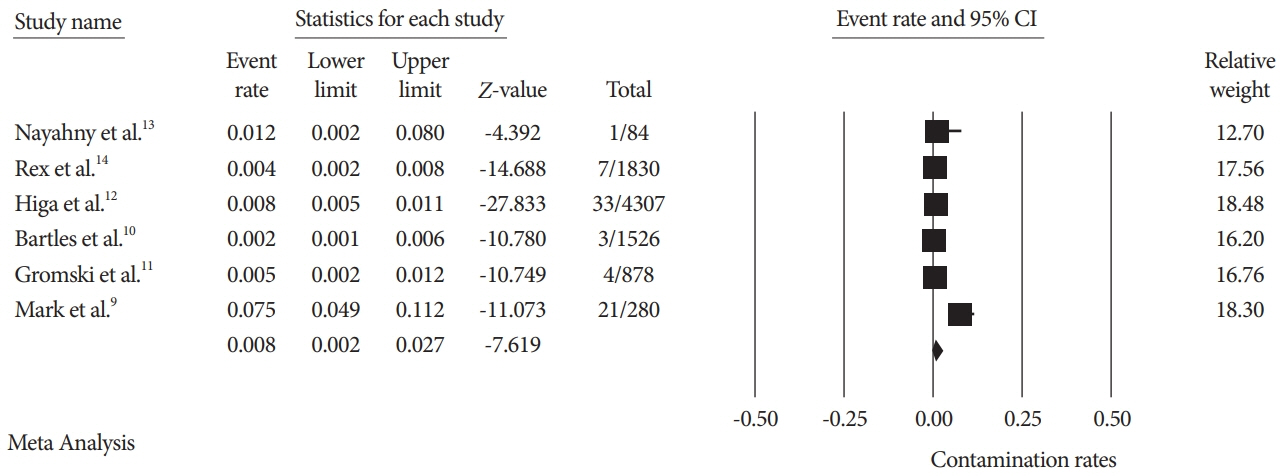
A total of seven studies using various ESRTs were incorporated in the analysis, which included a total of 9,084 post-ESRT duodenoscope cultures. The pooled contamination rate of the post-ESRT duodenoscope was 5% (95% confidence interval [CI]: 2.3%–10.8%, inconsistency index [I2]=97.97%). Pooled contamination rates for high-risk organisms were 0.8% (95% CI: 0.2%–2.7%, I2=94.96).
Conclusions
While ESRT may improve the disinfection process, a post-ESRT contamination rate of 5% is not negligible. Ongoing efforts to mitigate the rate of contamination by improving disinfection techniques and innovations in duodenoscope design to improve safety are warranted.
Keyword
Figure
Reference
-
1. FDA. Reducing the risk of infection from reprocessed duodenoscopes. [Internet]. Silver Spring: FDA;c2019. [cited 2021 Oct 13]. Available from: https://www.fda.gov/media/132187/download.2. Murray P, Member R. Health, Education, Labor, Pensions Committee. Preventable tragedies: superbugs and how ineffective monitoring of medical device safety fails patients. [Internet]. United States Senate Minority Staff Report;c2016. [cited 2021 Oct 13]. Available from: https://www.help.senate.gov/imo/media/doc/Duodenoscope%20Investigation%20FINAL%20Report.pdf.3. FDA. Design of endoscopic retrograde cholangiopancreatography (ERCP) duodenoscopes may impede effective cleaning: FDA safety communication [Internet]. Silver Spring: FDA;c2015. [cited 2021 Oct 13]. Available from: http://wayback.archive-it.org/7993/20170722213105/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm434871.htm.4. FDA. Supplemental measures to enhance duodenoscope reprocessing: FDA safety communication [Internet]. Silver Spring: FDA;c2015. [cited 2021 Oct 13]. Available from: http://wayback.archive-it.org/7993/20170722150658/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454766.htm.5. FDA. 522 Postmarket Surveillance Studies Database [Internet]. Silver Spring: FDA;c2016. [cited 2021 Oct 13]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=355&c_id=3727.6. FDA. 522 Postmarket Surveillance Studies Database [Internet]. Silver Spring: FDA;c2016. [cited 2021 Oct 13]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm?t_id=354&c_id=3726.7. Rauwers AW, Voor In ’t Holt AF, Buijs JG, et al. High prevalence rate of digestive tract bacteria in duodenoscopes: a nationwide study. Gut. 2018; 67:1637–1645.8. Ross AS, Baliga C, Verma P, Duchin J, Gluck M. A quarantine process for the resolution of duodenoscope-associated transmission of multidrug-resistant escherichia coli. Gastrointest Endosc. 2015; 82:477–483.9. Mark JA, Underberg K, Kramer RE. Results of duodenoscope culture and quarantine after manufacturer-recommended cleaning process. Gastrointest Endosc. 2020; 91:1328–1333.10. Bartles RL, Leggett JE, Hove S, et al. A randomized trial of single versus double high-level disinfection of duodenoscopes and linear echoendoscopes using standard automated reprocessing. Gastrointest Endosc. 2018; 88:306–313.e2.11. Gromski MA, Sieber MS, Sherman S, Rex DK. Double high-level disinfection versus liquid chemical sterilization for reprocessing of duodenoscopes used for ERCP: a prospective randomized study. Gastrointest Endosc. 2021; 93:927–931.12. Higa JT, Choe J, Tombs D, Gluck M, Ross AS. Optimizing duodenoscope reprocessing: rigorous assessment of a culture and quarantine protocol. Gastrointest Endosc. 2018; 88:223–229.13. Naryzhny I, Silas D, Chi K. Impact of ethylene oxide gas sterilization of duodenoscopes after a carbapenem-resistant Enterobacteriaceae outbreak. Gastrointest Endosc. 2016; 84:259–262.14. Rex DK, Sieber M, Lehman GA, et al. A double-reprocessing high-level disinfection protocol does not eliminate positive cultures from the elevators of duodenoscopes. Endoscopy. 2018; 50:588–596.15. Snyder GM, Wright SB, Smithey A, et al. Randomized comparison of 3 high-level disinfection and sterilization procedures for duodenoscopes. Gastroenterology. 2017; 153:1018–1025.16. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In : Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [Internet]. London: Cochrane;c2011. [updated 2011 Mar]. Available from: http://handbook-5-1.cochrane.org/.17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269. W64.18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605.19. FDA/CDC/ASM Working Group on Duodenoscope Culturing. Duodenoscope surveillance sampling and culturing protocols [Internet]. Silver Spring: FDA;c2018. [cited 2021 Oct 13]. Available from: https://www.fda.gov/media/111081/download.20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986; 7:177–188.21. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J Clin Epidemiol. 2011; 64:1294–1302.22. FDA. The FDA continues to remind facilities of the importance of following duodenoscope reprocessing instructions: FDA safety communication [Internet]. Silver Spring: FDA;c2019. [cited 2021 Oct 13]; Available from: https://www.fda.gov/medical-devices/safety-communications/fda-continues-remind-facilities-importance-following-duodenoscope-reprocessing-instructions-fda.23. Larsen S, Russell RV, Ockert LK, et al. Rate and impact of duodenoscope contamination: a systematic review and meta-analysis. EClinicalMedicine. 2020; 25:100451.24. FDA. The FDA is recommending transition to duodenoscopes with innovative designs to enhance safety: FDA Safety Communication [Internet]. Silver Spring: FDA;c2019. [cited 2021 Oct 13]; Available from: https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication.25. Pasricha PJ, Miller S, Carter F, Humphries R. Novel and effective disposable device that provides 2-way protection to the duodenoscope from microbial contamination. Gastrointest Endosc. 2020; 92:199–208.26. Ross AS, Bruno MJ, Kozarek RA, et al. Novel single-use duodenoscope compared with 3 models of reusable duodenoscopes for ERCP: a randomized bench-model comparison. Gastrointest Endosc. 2020; 91:396–403.27. Bang JY, Hawes R, Varadarajulu S. Equivalent performance of single-use and reusable duodenoscopes in a randomised trial. Gut. 2021; 70:838–844.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Single-use endoscopes: A narrative review
- Current Issues in Duodenoscope-Associated Infections: Now Is the Time to Take Action
- An Introduction of the Systematic Review and Meta-Analysis
- Systematic Review and Meta-analysis in Digestive Cancer Research
- Risk Factors for Tumor Size Increase During Active Surveillance of Papillary Thyroid Cancer: Meta-Analysis and Systematic Review