J Yeungnam Med Sci.
2022 Jan;39(1):24-30. 10.12701/yujm.2021.01165.
Magnetic resonance imaging texture analysis for the evaluation of viable ovarian tissue in patients with ovarian endometriosis: a retrospective case-control study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- KMID: 2525024
- DOI: http://doi.org/10.12701/yujm.2021.01165
Abstract
- Background
Texture analysis has been used as a method for quantifying image properties based on textural features. The aim of the present study was to evaluate the usefulness of magnetic resonance imaging (MRI) texture analysis for the evaluation of viable ovarian tissue on the perfusion map of ovarian endometriosis.
Methods
To generate a normalized perfusion map, subtracted T1-weighted imaging (T1WI), T1WI and contrast-enhanced T-WI with sequences were performed using the same parameters in 25 patients with surgically confirmed ovarian endometriosis. Integrated density is defined as the sum of the values of the pixels in the image or selection. We investigated the parameters for texture analysis in ovarian endometriosis, including angular second moment (ASM), contrast, correlation, inverse difference moment (IDM), and entropy, which is equivalent to the product of area and mean gray value.
Results
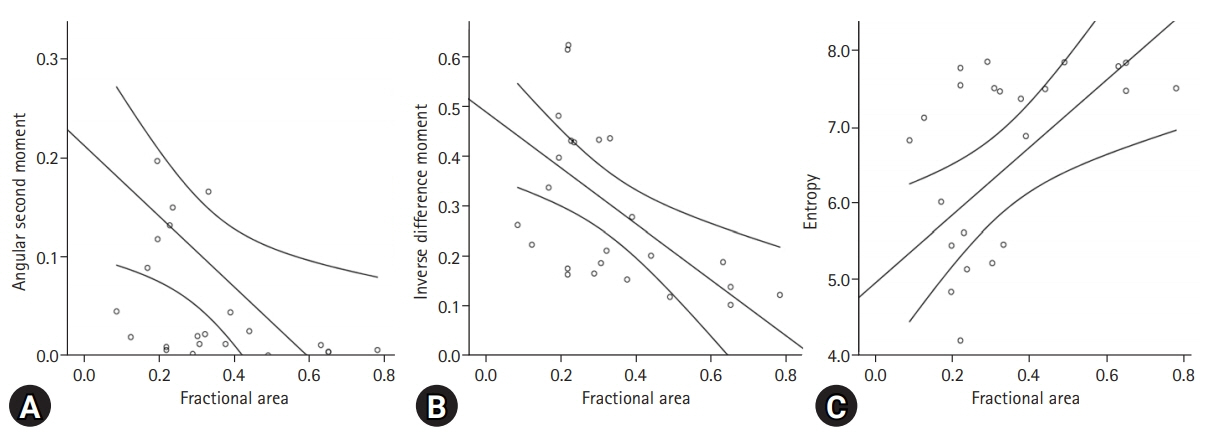
The perfusion ratio and integrated density of normal ovary were 0.52±0.05 and 238.72±136.21, respectively. Compared with the normal ovary, the affected ovary showed significant differences in total size (p<0.001), fractional area ratio (p<0.001), and perfusion ratio (p=0.010) but no significant differences in perfused tissue area (p=0.158) and integrated density (p=0.112). In comparison of parameters for texture analysis between the ovary with endometriosis and the contralateral normal ovary, ASM (p=0.004), contrast (p=0.002), IDM (p<0.001), and entropy (p=0.028) showed significant differences. A linear regression analysis revealed that fractional area had significant correlations with ASM (r2=0.211), IDM (r2=0.332), and entropy (r2=0.289).
Conclusion
Magnetic resonance texture analysis could be useful for the evaluation of viable ovarian tissues in patients with ovarian endometriosis.
Keyword
Figure
Reference
-
References
1. Yu JS, Rofsky NM. Dynamic subtraction MR imaging of the liver: advantages and pitfalls. AJR Am J Roentgenol. 2003; 180:1351–7.2. Hummelshoj L, Prentice A, Groothuis P. Update on endometriosis. Womens Health (Lond). 2006; 2:53–6.3. Working group of ESGE and WES, Saridogan E, Becker CM, Feki A, Grimbizis GF, Hummelshoj L, et al. Recommendations for the surgical treatment of endometriosis-part 1: ovarian endometrioma. Gynecol Surg. 2017; 14:27.4. Messinger Y, Yanishevski Y, Avramis VI, Ek O, Chelstrom LM, Gunther R, et al. Treatment of human B-cell precursor leukemia in SCID mice using a combination of the investigational biotherapeutic agent B43-PAP with cytosine arabinoside. Clin Cancer Res. 1996; 2:1533–42.5. Togashi K, Nakai A, Sugimura K. Anatomy and physiology of the female pelvis: MR imaging revisited. J Magn Reson Imaging. 2001; 13:842–9.6. Outwater EK, Mitchell DG. Normal ovaries and functional cysts: MR appearance. Radiology. 1996; 198:397–402.7. Woodward PJ, Sohaey R, Mezzetti TP Jr. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001; 21:193–216.8. Sugimura K, Okizuka H, Imaoka I, Kaji Y, Takahashi K, Kitao M, et al. Pelvic endometriosis: detection and diagnosis with chemical shift MR imaging. Radiology. 1993; 188:435–8.9. Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. 2014; 20:217–30.10. Togashi K, Nishimura K, Kimura I, Tsuda Y, Yamashita K, Shibata T, et al. Endometrial cysts: diagnosis with MR imaging. Radiology. 1991; 180:73–8.11. Herlidou-Même S, Constans JM, Carsin B, Olivie D, Eliat PA, Nadal-Desbarats L, et al. MRI texture analysis on texture test objects, normal brain and intracranial tumors. Magn Reson Imaging. 2003; 21:989–93.12. Bazot M, Bharwani N, Huchon C, Kinkel K, Cunha TM, Guerra A, et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol. 2017; 27:2765–75.13. Forstner R, Meissnitzer MW, Schlattau A, Spencer JA. MRI in ovarian cancer. Imaging Med. 2012; 4:59–75.14. Hornstein MD, Gleason RE, Orav J, Haas ST, Friedman AJ, Rein MS, et al. The reproducibility of the revised American Fertility Society classification of endometriosis. Fertil Steril. 1993; 59:1015–21.15. Schultes G. Klassifikation der Endometriose [Classification of endometriosis]. Wien Med Wochenschr. 1999; 149:361–5.16. Lee HJ. Usefulness of subtraction pelvic magnetic resonance imaging for detection of ovarian endometriosis. Yeungnam Univ J Med. 2020; 37:90–7.17. Grammatikakis I, Evangelinakis N, Salamalekis G, Tziortzioti V, Samaras C, Chrelias C, et al. Prevalence of severe pelvic inflammatory disease and endometriotic ovarian cysts: a 7-year retrospective study. Clin Exp Obstet Gynecol. 2009; 36:235–6.18. Mathias JM, Tofts PS, Losseff NA. Texture analysis of spinal cord pathology in multiple sclerosis. Magn Reson Med. 1999; 42:929–35.19. Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004; 59:1061–9.20. Lupean RA, Ștefan PA, Csutak C, Lebovici A, Măluțan AM, Buiga R, et al. Differentiation of endometriomas from ovarian hemorrhagic cysts at magnetic resonance: the role of texture analysis. Medicina (Kaunas). 2020; 56:487.21. Chan JH, Peh WC, Tsui EY, Wong KP, Yuen MK. Three-dimensional time-of-flight subtraction angiography of subacute cerebral hemorrhage. AJR Am J Roentgenol. 2003; 181:242–4.22. Cheng B, Cai W, Sun C, Kang Y, Gong J. 3D bone subtraction CT angiography for the evaluation of intracranial aneurysms: a comparison study with 2D bone subtraction CT angiography and conventional non-subtracted CT angiography. Acta Radiol. 2015; 56:1127–34.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of subtraction pelvic magnetic resonance imaging for detection of ovarian endometriosis
- Histographic Analysis of Magnetic Resonance Imaging for the Evaluation of Ovarian Endometrial Invasion
- A Case of Ovarian Cellular Fibroma with Endometriosis
- Tamoxifen-associated polypoid endometriosis mimicking an ovarian neoplasm
- Management of endometriosis-related infertility: Considerations and treatment options