J Korean Med Sci.
2022 Jan;37(3):e23. 10.3346/jkms.2022.37.e23.
Analyses of Confirmed COVID-19 Cases Among Korean Military Personnel After Mass Vaccination
- Affiliations
-
- 1Department of Internal Medicine, Division of Infectious Diseases, Armed Forces Yangju Hospital, Yangju, Korea
- 2Department of Internal Medicine, Division of Infectious Diseases, Armed Forces Capital Hospital, Seongnam, Korea
- 3Department of Laboratory Medicine, Armed Forces Medical Research Institute, Daejeon, Korea
- 4Department of Public Health Administration and Operation, Armed Forces Medical Command, Seongnam, Korea
- 5Armed Forces Medical Command, Seongnam, Korea
- 6Department of Critical Care Medicine, Department of Internal Medicine, Armed Forces Capital Hospital, Seongnam, Korea
- KMID: 2524892
- DOI: http://doi.org/10.3346/jkms.2022.37.e23
Abstract
- Background
The military was one of the first groups in Korea to complete mass vaccination against the coronavirus disease 2019 (COVID-19) due to their high vulnerability to COVID-19. To confirm the effect of mass vaccination, this study analyzed the patterns of confirmed cases within Korean military units.
Methods
From August 1 to September 15, 2021, all epidemiological data regarding confirmed COVID-19 cases in military units were reviewed. The number of confirmed cases in the units that were believed to have achieved herd immunity (i.e., ≥ 70% vaccination) was compared with the number of cases in the units that were not believed to have reached herd immunity (< 70% vaccination). Additionally, trends in the incidence rates of COVID-19 in the military and the entire Korean population were compared.
Results
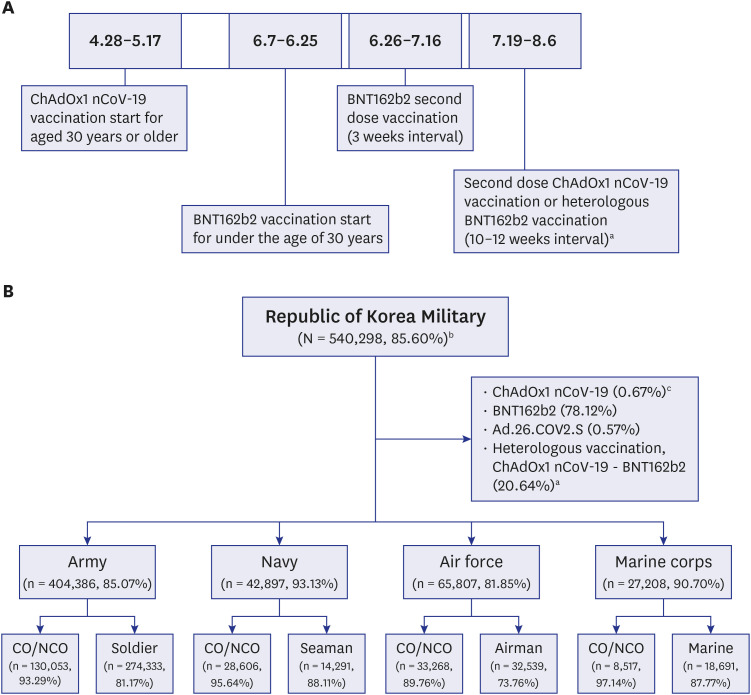
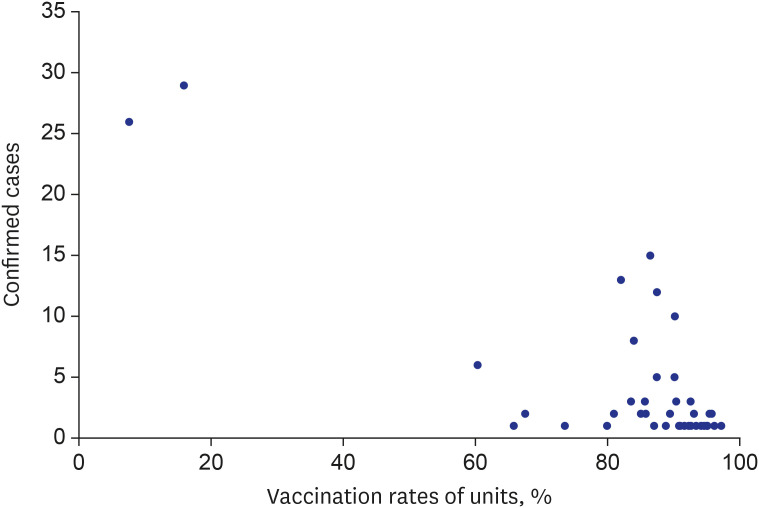
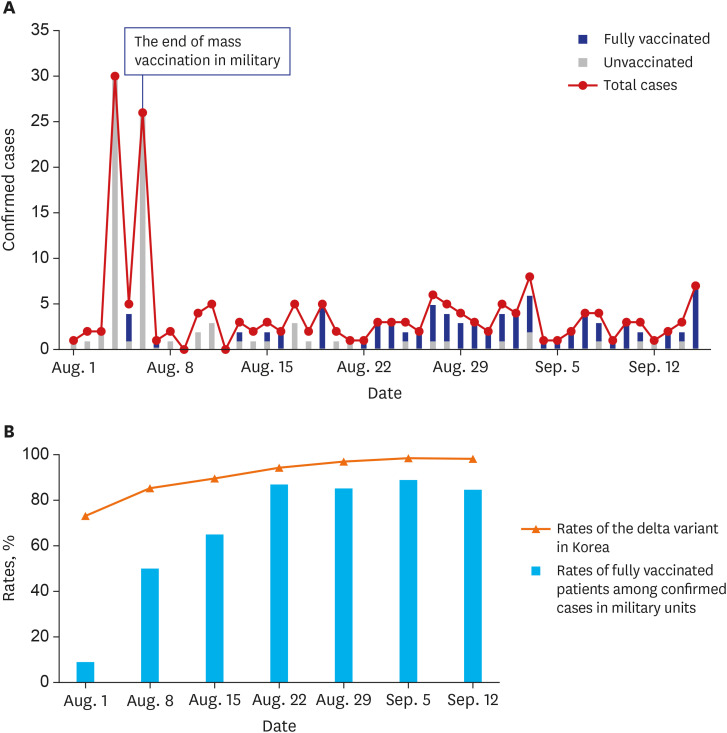
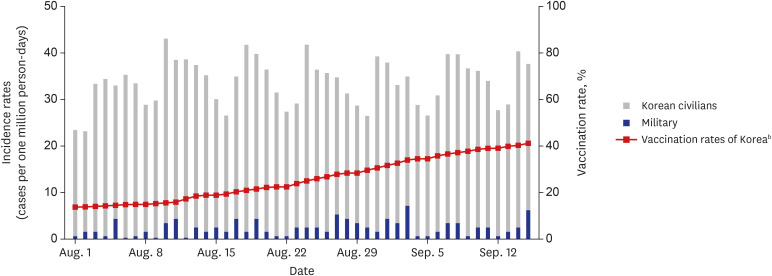
By August 2021, 85.60% of military personnel were fully vaccinated. During the study period, a total of 174 COVID-19 cases were confirmed in the 39 units. More local transmission (herd immunity group vs. non-herd immunity group [%], 1 [0.91] vs. 39 [60.94]) and hospitalizations (12 [11.01] vs. 13 [27.08]) occurred in the units that were not believed to have achieved herd immunity. The percentage of fully vaccinated individuals among the confirmed COVID-19 cases increased over time, possibly due to the prevalence of the delta variant. Nevertheless, the incidence rate remained lower in military units than in the general Korean population.
Conclusion
After completing mass vaccination, the incidence rates of COVID-19 infection in the military were lower than those in the national population. New cluster infections did not occur in vaccinated units, thereby suggesting that herd immunity has been achieved in these military units. Further research is needed to determine the extent to which levels of nonpharmacological intervention can be reduced in the future.
Keyword
Figure
Cited by 2 articles
-
Multi-Faceted Analysis of COVID-19 Epidemic in Korea Considering Omicron Variant: Mathematical Modeling-Based Study
Youngsuk Ko, Victoria May Mendoza, Renier Mendoza, Yubin Seo, Jacob Lee, Jonggul Lee, Donghyok Kwon, Eunok Jung
J Korean Med Sci. 2022;37(26):e209. doi: 10.3346/jkms.2022.37.e209.Trends in Confirmed COVID-19 Cases in the Korean Military Before and After the Emergence of the Omicron Variant
Dong Hoon Shin, Haebong Jang, Sangho Lee, Byung Seop Choi, Donghoon Kim, Hong Sang Oh
J Korean Med Sci. 2022;37(34):e260. doi: 10.3346/jkms.2022.37.e260.
Reference
-
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. PMID: 31978945.
Article2. Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021; 9(5):467. PMID: 34066475.
Article3. Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020; 52(5):737–741. PMID: 32433946.
Article4. Yoo JH. What we do know and do not yet know about COVID-19 vaccines as of the beginning of the year 2021. J Korean Med Sci. 2021; 36(6):e54–e50. PMID: 33559409.
Article5. Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel). 2021; 9(2):160. PMID: 33669441.
Article6. Wang R, Chen J, Gao K, Wei GW. Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics. 2021; 113(4):2158–2170. PMID: 34004284.
Article7. Ogawa J, Zhu W, Tonnu N, Singer O, Hunter T, Ryan AL, et al. The D614G mutation in the SARS-CoV2 Spike protein increases infectivity in an ACE2 receptor dependent manner. bioRxiv. July 22, 2020. DOI: 10.1101/2020.07.21.214932.
Article8. Gupta N, Kaur H, Yadav PD, Mukhopadhyay L, Sahay RR, Kumar A, et al. Clinical Characterization and genomic analysis of samples from COVID-19 breakthrough infections during the second wave among the various states of India. Viruses. 2021; 13(9):1782. PMID: 34578363.
Article9. Korea Disease Control and Prevention Agency (KDCA). Current status and characteristics of COVID-19 variants in July 2021 in Korea (9.2) (Korean). Updated 2021. Accessed October 24, 2021. https://kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=716753&act=view .10. Korea Disease Control and Prevention Agency (KDCA). COVID-19 Domestic occurrences report (Korean). Updated 2021. Accessed October 24, 2021. http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_id=&gubun .11. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021; 385(7):585–594. PMID: 34289274.12. Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance - nine states, June-August 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(37):1291–1293. PMID: 34529642.
Article13. Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus pandemic (COVID-19). Updated 2021. Accessed October 24, 2021. https://ourworldindata.org/coronavirus .14. Korean Food and Drug Administration (KFDA). Status of official approval for COVID-19 diagnostic reagents in Korea (Korean). Updated 2021. Accessed October 24, 2021. https://www.mfds.go.kr/brd/m_74/view.do?seq=44112 .15. Centers for Disease Control and Prevention (CDC). When you’ve been fully vaccinated. Updated 2021. Accessed October 24, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html#footnote .16. Park PG, Kim CH, Heo Y, Kim TS, Park CW, Kim CH. Out-of-hospital cohort treatment of coronavirus disease 2019 patients with mild symptoms in Korea: an experience from a single community treatment center. J Korean Med Sci. 2020; 35(13):e140–e140. PMID: 32242347.
Article17. Choe PG, Kang EK, Lee SY, Oh B, Im D, Lee HY, et al. Selecting coronavirus disease 2019 patients with negligible risk of progression: early experience from non-hospital isolation facility in Korea. Korean J Intern Med (Korean Assoc Intern Med). 2020; 35(4):765–770.
Article18. Jung J. Preparing for the coronavirus disease (COVID-19) vaccination: evidence, plans, and implications. J Korean Med Sci. 2021; 36(7):e59. PMID: 33619920.
Article19. Huh K, Na Y, Kim YE, Radnaabaatar M, Peck KR, Jung J. Predicted and observed incidence of thromboembolic events among Koreans vaccinated with ChAdOx1 nCoV-19 vaccine. J Korean Med Sci. 2021; 36(27):e197. PMID: 34254476.
Article20. Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021; 397(10277):881–891. PMID: 33617777.21. Korea Disease Control and Prevention Agency (KDCA). COVID-19 guidelines for local governments (Korean). 10th ed. Updated 2021. Accessed December 8, 2021. https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019&act=view&list_no=717651 .22. Korea Disease Control and Prevention Agency (KDCA). The detection rates of COVID-19 variants in Korea (Korean). Updated 2021. Accessed October 24, 2021. http://www.kdca.go.kr/contents.es?mid=a20107040000 .23. Ministry of Health and Welfare. Adjustment to the Social Distancing Scheme (11.29). Updated 2021. Accessed December 8, 2021. http://www.mohw.go.kr/eng/nw/nw0101vw.jsp?PAR_MENU_ID=1007&MENU_ID=100701&page=1&CONT_SEQ=368630&SEARCHKEY=CONTENT&SEARCHVALUE=pass .24. Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature. 1985; 318(6044):323–329. PMID: 3906406.
Article25. Eichner M, Schwehm M, Eichner L, Gerlier L. Direct and indirect effects of influenza vaccination. BMC Infect Dis. 2017; 17(1):308. PMID: 28441935.
Article26. Li B, Deng A, Li K, Hu Y, Li Z, Xiong Q, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 delta variant. medRxiv. July 23, 2021. DOI: 10.1101/2021.07.07.21260122.
Article27. Jung J. A long way to the recovery: COVID-19 will not disappear. J Korean Med Sci. 2021; 36(32):e231–e230. PMID: 34402229.
Article28. Li Y, Tenchov R, Smoot J, Liu C, Watkins S, Zhou Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci. 2021; 7(4):512–533. PMID: 34056083.
Article29. Barnett-Howell Z, Watson OJ, Mobarak AM. The benefits and costs of social distancing in high- and low-income countries. Trans R Soc Trop Med Hyg. 2021; 115(7):807–819. PMID: 33440007.
Article30. Asahi K, Undurraga EA, Valdés R, Wagner R. The effect of COVID-19 on the economy: Evidence from an early adopter of localized lockdowns. J Glob Health. 2021; 11:05002. PMID: 33643635.
Article31. Ministry of Health and Welfare. South Korea announces the roadmap for gradual return to normal (10.29) (Korean). Updated 2021. Accessed November 1, 2021. https://www.mohw.go.kr/eng/nw/nw0101vw.jsp?PAR_MENU_ID=1007&MENU_ID=100701&page=1&CONT_SEQ=368308 .32. COVID-19 Treatment Guidelines Panel (National Institutes of Health). Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated 2021. Accessed October 24, 2021. https://www.covid19treatmentguidelines.nih.gov/ .33. Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021; 398(10295):121–130. PMID: 34181880.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trends in Confirmed COVID-19 Cases in the Korean Military Before and After the Emergence of the Omicron Variant
- Qualitative Analysis of the Tetanus Antibody in Korean Army personnel after Visiting a Tertiary Armed Forces Hospital
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- Examination of Predicting Factors for COVID-19 Vaccination Behaviors of University Students Utilizing the Theory of Planned Behavior
- Vestibular Neuritis after COVID-19 Vaccination