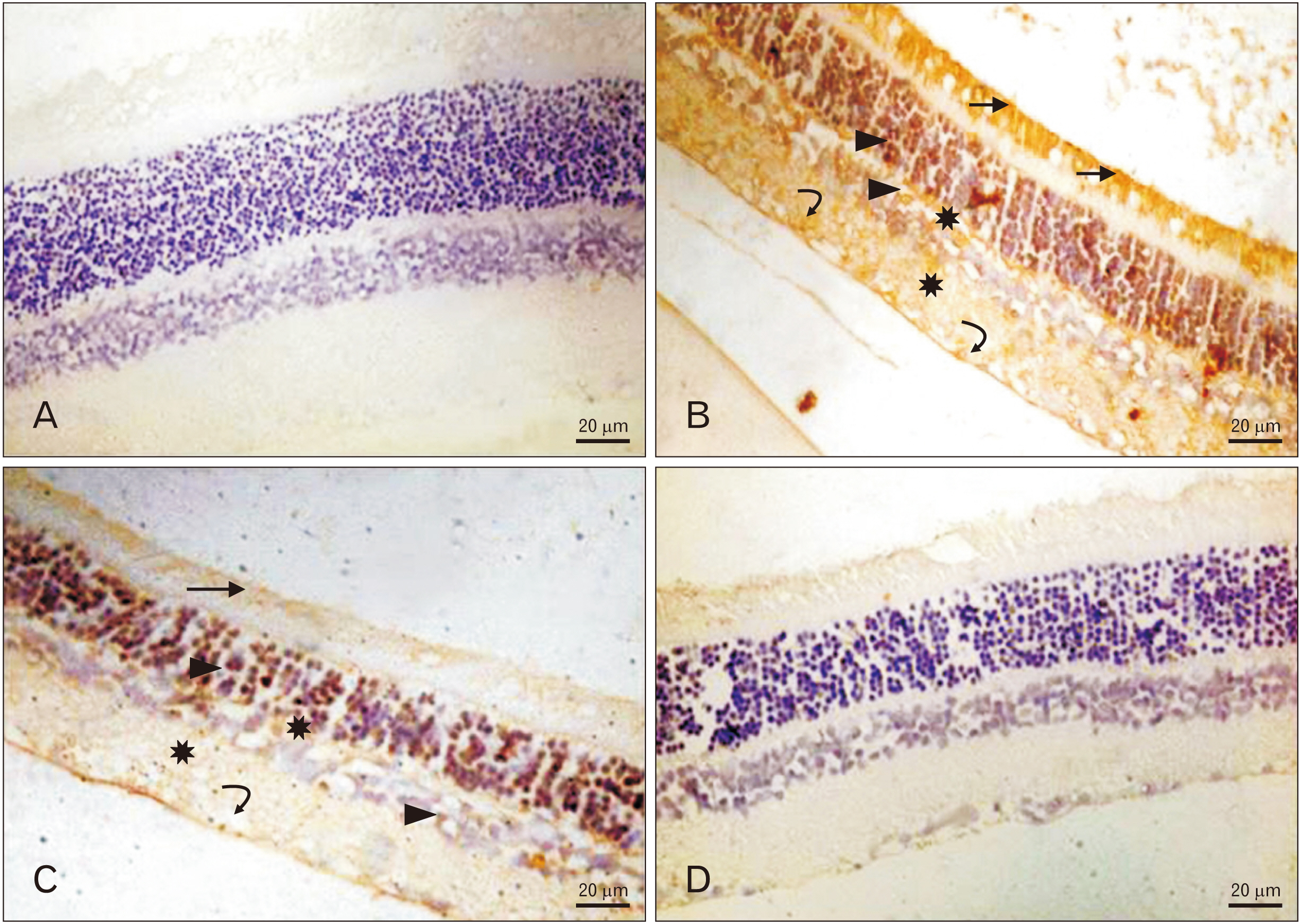
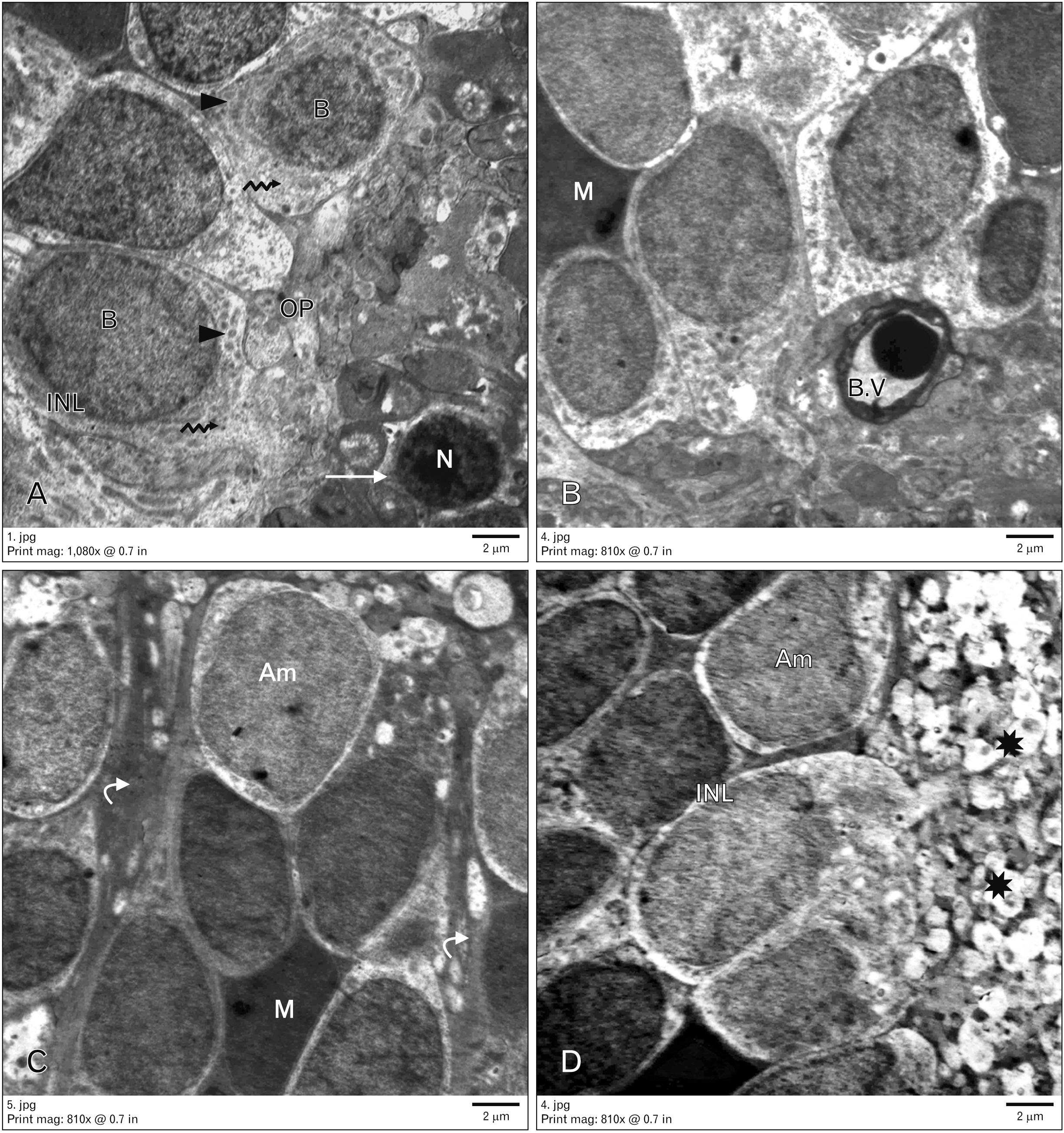
Anat Cell Biol.
2021 Dec;54(4):465-478. 10.5115/acb.21.105.
The role of hesperidin in ameliorating retinal changes in rats with experimentally induced type 1 diabetes mellitus and the active role of vascular endothelial growth factor and glial fibrillary acidic protein
- Affiliations
-
- 1Department of Histology and Cell Biology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
- 2Department of Histology and Cell Biology, Faculty of Medicine, Badr University in Cairo, Cairo, Egypt
- 3Ophthalmology Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 4Department of Biochemistry and Molecular Biology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
- KMID: 2523577
- DOI: http://doi.org/10.5115/acb.21.105
Abstract
- Patients with type 1 diabetes mellitus (T 1 DM) are vulnerable to developing diabetic retinopathy even under insulin therapy. Thus, this study was designed to evaluate the efficacy of hesperidin and insulin in rats with T 1 DM compared with insulin alone in improving diabetic retinal changes. Eighty rats were divided into four equal groups: group I, control rats without diabetes; group II, untreated rats with diabetes; group III, rats with diabetes treated daily with subcutaneous (SC) doses of long-acting insulin; and group IV, a rat with diabetes in which hesperidin was orally administered with SC insulin. The animals were assessed histologically, morphometrically, and biochemically. In group II, the thickness of all retinal layers decreased histologically. Ultrastructurally, degenerated retinal neurons and congested blood vessels were observed. Immunostaining detected elevated gene expression of advanced glycation end products. Gene expression of vascular endothelial growth factor, and glial fibrillary acidic protein were elevated. In this study, hesperidin supplementation with insulin significantly improved the retinal histological changes, supported by morphometric findings, compared with insulin alone. Moreover, treatment with hesperidin significantly reduced malondialdehyde and elevated serum antioxidant markers, including superoxide dismutase and catalase; furthermore, glutathione peroxidase decreased. Hesperidin might be an effective supplement for improving diabetic retinal complications occurring even with insulin treatment.
Keyword
Figure
Cited by 1 articles
-
Therapeutic effect of the mesenchymal stem cells on vigabatrin-induced retinopathy in adult male albino rat
Ayat Mahmoud Domouky, Walaa M. Samy, Walaa A. Rashad
Anat Cell Biol. 2022;55(2):217-228. doi: 10.5115/acb.22.006.
Reference
-
References
1. Mwangi MW, Githinji GG, Githinji FW. 2011; Knowledge and awareness of diabetic retinopathy amongst diabetic patients in Kenyatta National Hospital, Kenya. Int J Hum Soc Sci. 1:140–6.2. Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. 2013; Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013:343560. DOI: 10.1155/2013/343560. PMID: 24563789. PMCID: PMC3914226.
Article3. Altmann C, Schmidt MHH. 2018; The role of microglia in diabetic retinopathy: inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci. 19:110. DOI: 10.3390/ijms19010110. PMID: 29301251. PMCID: PMC5796059.
Article4. Grosso A, Cheung N, Veglio F, Wong TY. 2011; Similarities and differences in early retinal phenotypes in hypertension and diabetes. J Hypertens. 29:1667–75. DOI: 10.1097/HJH.0b013e3283496655. PMID: 21841544.
Article5. Sasongko MB, Wong TY, Nguyen TT, Kawasaki R, Jenkins A, Shaw J, Wang JJ. 2011; Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care. 34:474–9. DOI: 10.2337/dc10-0793. PMID: 21270203. PMCID: PMC3024371.
Article6. Kowluru RA, Chan PS. 2007; Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007:43603. DOI: 10.1155/2007/43603. PMID: 17641741. PMCID: PMC1880867.
Article7. Tahrani AA, Piya MK, Kennedy A, Barnett AH. 2010; Glycaemic control in type 2 diabetes: targets and new therapies. Pharmacol Ther. 125:328–61. DOI: 10.1016/j.pharmthera.2009.11.001. PMID: 19931305.
Article8. Li WQ, Park Y, Wu JW, Ren JS, Goldstein AM, Taylor PR, Hollenbeck AR, Freedman ND, Abnet CC. 2013; Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol. 11:1130–6.e2. DOI: 10.1016/j.cgh.2013.03.023. PMID: 23591281. PMCID: PMC3758458.
Article9. Yari Z, Movahedian M, Imani H, Alavian SM, Hedayati M, Hekmatdoost A. 2020; The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. Eur J Nutr. 59:2569–77. DOI: 10.1007/s00394-019-02105-2. PMID: 31844967.
Article10. Kawaguchi K, Mizuno T, Aida K, Uchino K. 1997; Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci Biotechnol Biochem. 61:102–4. DOI: 10.1271/bbb.61.102. PMID: 9028038.11. Akiyama S, Katsumata S, Suzuki K, Ishimi Y, Wu J, Uehara M. 2010; Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr. 46:87–92. DOI: 10.3164/jcbn.09-82. PMID: 20104270. PMCID: PMC2803138.
Article12. Kandhare AD, Mukherjee A, Bodhankar SL. 2017; Effect of hesperidin in bleomycin-induced pulmonary fibrosis in rats: critical role of NRF-2, TNF-Α, and IL-1Β. Value Health. 20:A887–8. DOI: 10.1016/j.jval.2017.08.2657.
Article13. National Research Council (U.S.), Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals. National Academy Press;Washington, D.C.: p. 21–55.14. Kohzaki K, Vingrys AJ, Bui BV. 2008; Early inner retinal dysfunction in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 49:3595–604. DOI: 10.1167/iovs.08-1679. PMID: 18421077.
Article15. Subramanian P, Anandan R, Jayapalan JJ, Hashim OH. 2015; Hesperidin protects gentamicin-induced nephrotoxicity via Nrf2/HO-1 signaling and inhibits inflammation mediated by NF-κB in rats. J Funct Foods. 13:89–99. DOI: 10.1016/j.jff.2014.12.035.
Article16. Foureaux G, Nogueira BS, Coutinho DC, Raizada MK, Nogueira JC, Ferreira AJ. 2015; Activation of endogenous angiotensin converting enzyme 2 prevents early injuries induced by hyperglycemia in rat retina. Braz J Med Biol Res. 48:1109–14. DOI: 10.1590/1414-431x20154583. PMID: 26421871. PMCID: PMC4661027.
Article17. Fröde TS, Medeiros YS. 2008; Animal models to test drugs with potential antidiabetic activity. J Ethnopharmacol. 115:173–83. DOI: 10.1016/j.jep.2007.10.038. PMID: 18068921.
Article18. Bancroft JD, Gamble M. 2002. Theory and practice of histological techniques. 5th ed. Churchill Livingstone;London:19. Kiernan JA. 2000. Histological and histochemical methods: theory and practice. 3rd ed. Butterworth Heinemann;Oxford:20. Kim J, Kim CS, Lee YM, Sohn E, Jo K, Kim JS. 2015; Litsea japonica extract inhibits neuronal apoptosis and the accumulation of advanced glycation end products in the diabetic mouse retina. Mol Med Rep. 12:1075–81. DOI: 10.3892/mmr.2015.3543. PMID: 25815519. PMCID: PMC4438968.
Article21. Glauert AM, Lewis PR. 1998. Biological specimen preparation for transmission electron microscopy. Portland Press;London: DOI: 10.1515/9781400865024.22. Woo TT, Li SY, Lai WW, Wong D, Lo AC. 2013; Neuroprotective effects of lutein in a rat model of retinal detachment. Graefes Arch Clin Exp Ophthalmol. 251:41–51. DOI: 10.1007/s00417-012-2128-z. PMID: 22899456. PMCID: PMC3536954.
Article23. Saenz-de-Viteri M, Heras-Mulero H, Fernández-Robredo P, Recalde S, Hernández M, Reiter N, Moreno-Orduña M, García-Layana A. 2014; Oxidative stress and histological changes in a model of retinal phototoxicity in rabbits. Oxid Med Cell Longev. 2014:637137. DOI: 10.1155/2014/637137. PMID: 24991304. PMCID: PMC4058492.
Article24. Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–8. DOI: 10.1006/meth.2001.1262. PMID: 11846609.25. Kashim RM, Newton P, Ojo O. 2018; Diabetic retinopathy screening: a systematic review on patients' non-attendance. Int J Environ Res Public Health. 15:157. DOI: 10.3390/ijerph15010157. PMID: 29351207. PMCID: PMC5800256.
Article26. Jiang T, Chang Q, Cai J, Fan J, Zhang X, Xu G. 2016; Protective effects of melatonin on retinal inflammation and oxidative stress in experimental diabetic retinopathy. Oxid Med Cell Longev. 2016:3528274. DOI: 10.1155/2016/3528274. PMID: 27143993. PMCID: PMC4837288.
Article27. Ali AM, Gabbar MA, Abdel-Twab SM, Fahmy EM, Ebaid H, Alhazza IM, Ahmed OM. 2020; Antidiabetic potency, antioxidant effects, and mode of actions of Citrus reticulata fruit peel hydroethanolic extract, hesperidin, and quercetin in nicotinamide/streptozotocin-induced Wistar diabetic rats. Oxid Med Cell Longev. 2020:1730492. DOI: 10.1155/2020/1730492. PMID: 32655759. PMCID: PMC7327566.28. Saxena Y, Purwar B, Meena H, Sarthi P. 2014; Dolichos biflorus Linn. ameliorates diabetic complications in streptozotocin induced diabetic rats. Ayu. 35:442–6. DOI: 10.4103/0974-8520.159022. PMID: 26195910. PMCID: PMC4492032.
Article29. Yang QH, Zhang Y, Jiang J, Wu MM, Han Q, Bo QY, Yu GW, Ru YS, Liu X, Huang M, Wang L, Zhang XM, Fang JM, Li XR. 2018; Protective effects of a novel drug RC28-E blocking both VEGF and FGF2 on early diabetic rat retina. Int J Ophthalmol. 11:935–44. DOI: 10.18240/ijo.2018.06.07. PMID: 29977804. PMCID: PMC6010370.
Article30. Roy S, Trudeau K, Roy S, Tien T, Barrette KF. 2013; Mitochondrial dysfunction and endoplasmic reticulum stress in diabetic retinopathy: mechanistic insights into high glucose-induced retinal cell death. Curr Clin Pharmacol. 8:278–84. DOI: 10.2174/1574884711308040003. PMID: 23173958.
Article31. Ozdemir G, Ergün Y, Bakariş S, Kılınç M, Durdu H, Ganiyusufoğlu E. 2014; Melatonin prevents retinal oxidative stress and vascular changes in diabetic rats. Eye (Lond). 28:1020–7. DOI: 10.1038/eye.2014.127. PMID: 24924441. PMCID: PMC4135253.32. Toyoda F, Tanaka Y, Shimmura M, Kinoshita N, Takano H, Kakehashi A. 2016; Diabetic retinal and choroidal edema in SDT rats. J Diabetes Res. 2016:2345141. DOI: 10.1155/2016/2345141. PMID: 26783535. PMCID: PMC4691483.
Article33. Soufi FG, Mohammad-Nejad D, Ahmadieh H. 2012; Resveratrol improves diabetic retinopathy possibly through oxidative stress - nuclear factor κB - apoptosis pathway. Pharmacol Rep. 64:1505–14. DOI: 10.1016/S1734-1140(12)70948-9. PMID: 23406761.
Article34. Qian H, Ripps H. 2011; Neurovascular interaction and the pathophysiology of diabetic retinopathy. Exp Diabetes Res. 2011:693426. DOI: 10.1155/2011/693426. PMID: 21747832. PMCID: PMC3124285.
Article35. Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. 2005; Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 54:1559–65. DOI: 10.2337/diabetes.54.5.1559. PMID: 15855346.
Article36. Çerman E, Akkoç T, Eraslan M, Şahin Ö, Özkara S, Vardar Aker F, Subaşı C, Karaöz E, Akkoç T. 2016; Retinal electrophysiological effects of intravitreal bone marrow derived mesenchymal stem cells in streptozotocin induced diabetic rats. PLoS One. 11:e0156495. DOI: 10.1371/journal.pone.0156495. PMID: 27300133. PMCID: PMC4907488.
Article37. Liang X, Zhou H, Ding Y, Li J, Yang C, Luo Y, Li S, Sun G, Liao X, Min W. 2012; TMP prevents retinal neovascularization and imparts neuroprotection in an oxygen-induced retinopathy model. Invest Ophthalmol Vis Sci. 53:2157–69. DOI: 10.1167/iovs.11-9315. PMID: 22410554. PMCID: PMC4627509.
Article38. Chen W, Yao X, Zhou C, Zhang Z, Gui G, Lin B. 2017; Danhong Huayu Koufuye prevents diabetic retinopathy in streptozotocin-induced diabetic rats via antioxidation and anti-inflammation. Mediators Inflamm. 2017:3059763. DOI: 10.1155/2017/3059763. PMID: 28638179. PMCID: PMC5468776.
Article39. Yamagishi S, Inagaki Y, Amano S, Okamoto T, Takeuchi M, Makita Z. 2002; Pigment epithelium-derived factor protects cultured retinal pericytes from advanced glycation end product-induced injury through its antioxidative properties. Biochem Biophys Res Commun. 296:877–82. DOI: 10.1016/S0006-291X(02)00940-3. PMID: 12200129.
Article40. Raghu G, Akileshwari C, Reddy VS, Reddy GB. 2017; Attenuation of diabetic retinopathy in rats by ellagic acid through inhibition of AGE formation. J Food Sci Technol. 54:2411–21. DOI: 10.1007/s13197-017-2683-8. PMID: 28740299. PMCID: PMC5502036.
Article41. Kuo JZ, Wong TY, Rotter JI. 2014; Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 132:96–107. DOI: 10.1001/jamaophthalmol.2013.5024. PMID: 24201651. PMCID: PMC3947937.
Article42. Xin X, Li Y, Liu H. 2020; Hesperidin ameliorates hypobaric hypoxia-induced retinal impairment through activation of Nrf2/HO-1 pathway and inhibition of apoptosis. Sci Rep. 10:19426. DOI: 10.1038/s41598-020-76156-5. PMID: 33173100. PMCID: PMC7655840.
Article43. Maekawa S, Sato K, Fujita K, Daigaku R, Tawarayama H, Murayama N, Moritoh S, Yabana T, Shiga Y, Omodaka K, Maruyama K, Nishiguchi KM, Nakazawa T. 2017; The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci Rep. 7:6885. DOI: 10.1038/s41598-017-06969-4. PMID: 28761134. PMCID: PMC5537259.
Article44. Liu WY, Liou SS, Hong TY, Liu IM. 2017; Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients. 9:1312. DOI: 10.3390/nu9121312. PMID: 29207476. PMCID: PMC5748762.
Article45. Kumar B, Gupta SK, inivasan BP Sr, Nag TC, ivastava S Sr, Saxena R, Jha KA. 2013; Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc Res. 87:65–74. DOI: 10.1016/j.mvr.2013.01.002. PMID: 23376836.
Article46. Shi X, Liao S, Mi H, Guo C, Qi D, Li F, Zhang C, Yang Z. 2012; Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Molecules. 17:12868–81. DOI: 10.3390/molecules171112868. PMID: 23117428. PMCID: PMC6268103.
Article47. Kara S, Gencer B, Karaca T, Tufan HA, Arikan S, Ersan I, Karaboga I, Hanci V. 2014; Protective effect of hesperetin and naringenin against apoptosis in ischemia/reperfusion-induced retinal injury in rats. ScientificWorldJournal. 2014:797824. DOI: 10.1155/2014/797824. PMID: 24616645. PMCID: PMC3925573.
Article48. Eltony SA, Mohaseb HS, Sayed MM, Ahmed AA. 2021; Metformin treatment confers protection of the optic nerve following photoreceptor degeneration. Anat Cell Biol. 54:249–58. DOI: 10.5115/acb.20.320. PMID: 34162765. PMCID: PMC8225472.
Article49. Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL. 2014; Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 52:814–28. DOI: 10.3109/13880209.2013.870584. PMID: 24559476.
Article50. Kakadiya J, Patel D, Shah N. 2010; Effect of hesperidin on renal complication in experimentally induced renal damage in diabetic Sprague dawley rats. J Ecobiotechnol. 2:45–50.51. Kowluru RA, Tang J, Kern TS. 2001; Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 50:1938–42. DOI: 10.2337/diabetes.50.8.1938. PMID: 11473058.52. Obrosova IG, Drel VR, Kumagai AK, Szábo C, Pacher P, Stevens MJ. 2006; Early diabetes-induced biochemical changes in the retina: comparison of rat and mouse models. Diabetologia. 49:2525–33. DOI: 10.1007/s00125-006-0356-7. PMID: 16896942. PMCID: PMC2228251.
Article53. Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, ivastava S Sr. 2011; Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 27:123–30. DOI: 10.1089/jop.2010.0123. PMID: 21314438.
Article54. Aranganathan S, Selvam JP, Sangeetha N, Nalini N. 2009; Modulatory efficacy of hesperetin (citrus flavanone) on xenobiotic-metabolizing enzymes during 1,2-dimethylhydrazine-induced colon carcinogenesis. Chem Biol Interact. 180:254–61. DOI: 10.1016/j.cbi.2009.03.005. PMID: 19497424.
Article55. Aranganathan S, Nalini N. 2009; Efficacy of the potential chemopreventive agent, hesperetin (citrus flavanone), on 1,2-dimethylhydrazine induced colon carcinogenesis. Food Chem Toxicol. 47:2594–600. DOI: 10.1016/j.fct.2009.07.019. PMID: 19632289.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- IL-17A exacerbates diabetic retinopathy by impairing Müller cell function via Act1 signaling
- Expression of c-fos, p53, Transforming Growth Factor-beta1 and Glial Fibrillary Acidic Protein in Hippocampus Following Transient Forebrain Ischemia in Mongolian Gerbil
- A Case of Myxoid Neurothekeoma on the Hand
- The Significance of Induction of Heat Shock Protein-70 and Glial Fibrillary Acidic Protein Messenger RNA by Delayed Postischemic Hyperthermia Following Transient Focal Ischemia
- Profiles of 7-kDa Stress Protein, betaAPP, Proliferating Cell Nuclear Antigen, and Glial Fibrillary Acidic Protein in the Gerbil with Focal Infarction and Combined Focal Infarction and Global Ischemia: Relation with Ischemic Tolerance