Anat Cell Biol.
2021 Dec;54(4):448-464. 10.5115/acb.21.068.
Role of nuclear factor-kappa B in bleomycin induced pulmonary fibrosis and the probable alleviating role of ginsenoside: histological, immunohistochemical, and biochemical study
- Affiliations
-
- 1Medical Histology and Cell Biology Department, Faculty of Medicine, Mansoura University, El Mansoura, Egypt
- KMID: 2523576
- DOI: http://doi.org/10.5115/acb.21.068
Abstract
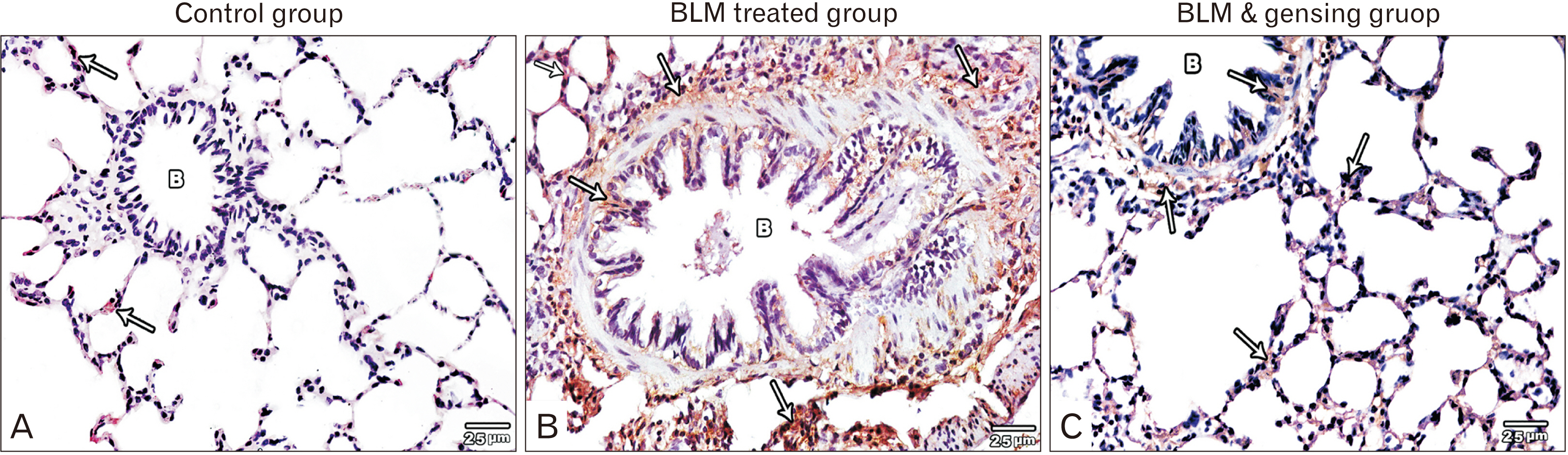
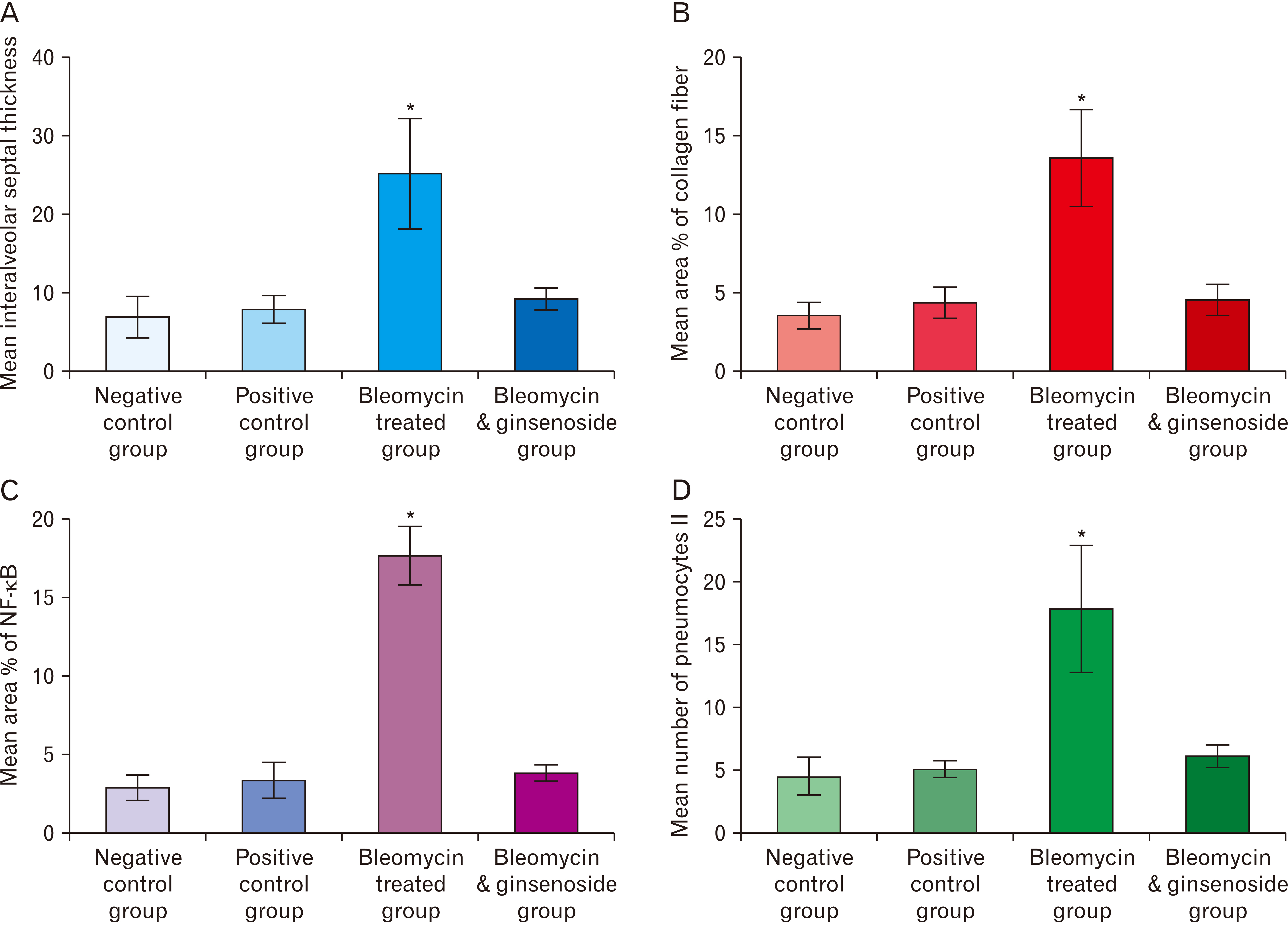
- Bleomycin (BLM) is one of anti-cancerous drugs. One of its limitation is the development of pulmonary fibrosis during therapy So, we proposed to examine the outcome of BLM take on the light and electron microscopic design of rat lung. Along with, assessment the probable protecting role of ginsenoside on BLM induced pulmonary changes. In this study, thirty adult male albino rats were comprised and were classified to four clusters; Negative & positive control group, BLM treated group and BLM& ginsenoside treated group. The lung was treated for histological and immunohistochemical (anti-p65) studies. Light microscopic examination of H&E stained sections of BLM treated group showed huge distortion of the lung building. Mallory trichrome stain of this group showed evident deposition of collagen fibers in the markedly thickened interalveolar septa and around intrapulmonary bronchi, bronchioles and blood vessels. Moreover, strong positive staining for nuclear factor (NF)-κB in the wall of bronchiole as well as the thickened interalveolar septa were observed. Ultrastructural inspection of lung of this group revealed muddled lung planning. Marked improvement of the lung structure and marked reduction in NF-κB immunoexpression was appeared in BLM and ginsenoside treated group. So, we concluded that coadministration of ginsenoside with BLM significantly enhanced the histological and morphometric image of the lung.
Figure
Cited by 1 articles
-
Tamarindus indica ameliorates behavioral and cytoarchitectural changes in the cerebellar cortex following prenatal aluminum chloride exposure in Wistar rats
Ibe Michael Usman, Samuel Sunday Adebisi, Sunday Abraham Musa, Ibrahim Abdullahi Iliya, Victor Bassey Archibong, Ann Monima Lemuel, Keneth Iceland Kasozi
Anat Cell Biol. 2022;55(3):320-329. doi: 10.5115/acb.22.033.
Reference
-
References
1. Albera C, Costabel U, Fagan EA, Glassberg MK, Gorina E, Lancaster L, Lederer DJ, Nathan SD, Spirig D, Swigris JJ. 2016; Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur Respir J. 48:843–51. DOI: 10.1183/13993003.01966-2015. PMID: 27471208.
Article2. Liu Y, Lu F, Kang L, Wang Z, Wang Y. 2017; Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 17:63. DOI: 10.1186/s12890-017-0405-7. PMID: 28420366. PMCID: PMC5395978.
Article3. Cui Y, Jiang L, Yu R, Shao Y, Mei L, Tao Y. 2019; β-carboline alkaloids attenuate bleomycin induced pulmonary fibrosis in mice through inhibiting NF-kb/p65 phosphorylation and epithelial-mesenchymal transition. J Ethnopharmacol. 243:112096. DOI: 10.1016/j.jep.2019.112096. PMID: 31323300.
Article4. Stoll P, Wuertemberger U, Bratke K, Zingler C, Virchow JC, Lommatzsch M. 2012; Stage-dependent association of BDNF and TGF-β1 with lung function in stable COPD. Respir Res. 13:116. DOI: 10.1186/1465-9921-13-116. PMID: 23245944. PMCID: PMC3561140.5. Choi SM, Jang AH, Kim H, Lee KH, Kim YW. 2016; Metformin reduces bleomycin-induced pulmonary fibrosis in mice. J Korean Med Sci. 31:1419–25. DOI: 10.3346/jkms.2016.31.9.1419. PMID: 27510385. PMCID: PMC4974183.
Article6. Wang M, Zhang J, Song X, Liu W, Zhang L, Wang X, Lv C. 2013; Astaxanthin ameliorates lung fibrosis in vivo and in vitro by preventing transdifferentiation, inhibiting proliferation, and promoting apoptosis of activated cells. Food Chem Toxicol. 56:450–8. DOI: 10.1016/j.fct.2013.03.004. PMID: 23500768.7. Peng R, idhar S Sr, Tyagi G, Phillips JE, Garrido R, Harris P, Burns L, Renteria L, Woods J, Chen L, Allard J, Ravindran P, Bitter H, Liang Z, Hogaboam CM, Kitson C, Budd DC, Fine JS, Bauer CM, Stevenson CS. 2013; Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for "active" disease. PLoS One. 8:e59348. DOI: 10.1371/journal.pone.0059348. PMID: 23565148. PMCID: PMC3614979.
Article8. Yao Y, Wang Y, Zhang Z, He L, Zhu J, Zhang M, He X, Cheng Z, Ao Q, Cao Y, Yang P, Su Y, Zhao J, Zhang S, Yu Q, Ning Q, Xiang X, Xiong W, Wang CY, Xu Y. 2016; Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol Ther. 24:915–25. DOI: 10.1038/mt.2016.36. PMID: 26883801. PMCID: PMC4881771.
Article9. Gerson SL, Caimi PF, William BM, Creger RJ. Hoffman R, Benz EJ, Silberstein LE, Heslop H, Weitz J, Anastasi J, Salama ME, Abutalib SA, editors. 2018. Pharmacology and molecular mechanisms of antineoplastic agents for hematologic malignancies. Hematology: Basic Principles and Practice. 7th ed. Elsevier Health Sciences;Philadelphia: p. 849–912. DOI: 10.1016/B978-0-323-35762-3.00057-3.
Article10. Shariati S, Kalantar H, Pashmforoosh M, Mansouri E, Khodayar MJ. 2019; Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed Pharmacother. 114:108776. DOI: 10.1016/j.biopha.2019.108776. PMID: 30903918.
Article11. Ballington DA, Anderson RJ. 2014. Pharmacy practice for technicians. 5th ed. Paradigm Publishing;St. Paul:12. Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H. 2018; Medicinal plants: Past history and future perspective. J Herbmed Pharmacol. 7:1–7. DOI: 10.15171/jhp.2018.01.
Article13. Shergis J, Di Y, Zhang A, Vlahos R, Helliwell R, Ye J, Xue CC. 2014; Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement Ther Med. 22:944–53. DOI: 10.1016/j.ctim.2014.08.006. PMID: 25440386.
Article14. Niranjana Murthy H, Dandin VS, Yoeup Paek K. 2014; Hepatoprotective activity of ginsenosides from Panax ginseng adventitious roots against carbon tetrachloride treated hepatic injury in rats. J Ethnopharmacol. 158 Pt A:442–6. DOI: 10.1016/j.jep.2014.10.047. PMID: 25446594.
Article15. Bao S, Zou Y, Wang B, Li Y, Zhu J, Luo Y, Li J. 2015; Ginsenoside Rg1 improves lipopolysaccharide-induced acute lung injury by inhibiting inflammatory responses and modulating infiltration of M2 macrophages. Int Immunopharmacol. 28:429–34. DOI: 10.1016/j.intimp.2015.06.022. PMID: 26122136.
Article16. Nguyen CT, Luong TT, Lee SY, Kim GL, Kwon H, Lee HG, Park CK, Rhee DK. 2015; Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine. 22:1055–61. DOI: 10.1016/j.phymed.2015.07.005. PMID: 26407948.17. Wang CZ, Anderson S, DU W, He TC, Yuan CS. 2016; Red ginseng and cancer treatment. Chin J Nat Med. 14:7–16. DOI: 10.3724/SP.J.1009.2016.00007. PMID: 26850342.18. Chen J, Feng X, Huang Q. 2016; Modulation of T-Bet and GATA-3 expression in experimental autoimmune thyroiditis rats through ginsenoside treatment. Endocr Res. 41:28–33. DOI: 10.3109/07435800.2015.1066800. PMID: 26523790.
Article19. Guan S, Liu Q, Han F, Gu W, Song L, Zhang Y, Guo X, Xu W. 2017; Ginsenoside Rg1 ameliorates cigarette smoke-induced airway fibrosis by suppressing the TGF-β1/Smad pathway in vivo and in vitro. Biomed Res Int. 2017:6510198. DOI: 10.1155/2017/6510198. PMID: 28421197. PMCID: PMC5379083.20. Behr J. 2013; The diagnosis and treatment of idiopathic pulmonary fibrosis. Dtsch Arztebl Int. 110:875–81. DOI: 10.3238/arztebl.2013.0875. PMID: 24529303. PMCID: PMC3928534.
Article21. Costabel U. 2015; The changing treatment landscape in idiopathic pulmonary fibrosis. Eur Respir Rev. 24:65–8. DOI: 10.1183/09059180.00011414. PMID: 25726557.
Article22. Zhou J, Zhang HA, Lin Y, Liu HM, Cui YM, Xu Y, Zhao N, Ma JM, Fan K, Jiang CL. 2014; Protective effect of ginsenoside against acute renal failure via reduction of renal oxidative stress and enhanced expression of ChAT in the proximal convoluted tubule and ERK1/2 in the paraventricular nuclei. Physiol Res. 63:597–604. DOI: 10.33549/physiolres.932721. PMID: 24908085.
Article23. Kamel EO, Hady AARA, Abd Elrahman ASAH, Diab MM. 2019; Evaluation of role of captopril and erdosteine in protection of the lung against bleomycin-induced injury in rats. Egypt J Hosp Med. 75:1937–45. DOI: 10.21608/ejhm.2019.29117.
Article24. Ma H, Jo YJ, Ma Y, Hong JT, Kwon BM, Oh KW. 2009; Obovatol isolated from Magnolia obovata enhances pentobarbital-induced sleeping time: possible involvement of GABAA receptors/chloride channel activation. Phytomedicine. 16:308–13. DOI: 10.1016/j.phymed.2008.12.007. PMID: 19201178.
Article25. Bancroft JD, Layton C. Suvarna SK, Layton C, Bancroft JD, editors. 2019. The hematoxylins and eosin. Bancroft's Theory and Practice of Histological Techniques. 8th ed. Elsevier;London: p. 126–38. DOI: 10.1016/B978-0-7020-6864-5.00010-4.
Article26. Kiernan JA. 2015. Histological and histochemical methods: theory and practice. 5th ed. Scion;Banbury:27. Sanderson T, Wild G, Cull AM, Marston J, Zardin G. Suvarna SK, Layton C, Bancroft JD, editors. 2019. Immunohistochemical and immunofluorescent techniques. Bancroft's Theory and Practice of Histological Techniques. 8th ed. Elsevier;London: p. 337–94. DOI: 10.1016/B978-0-7020-6864-5.00019-0.
Article28. Woods AE, Stirling JW. Suvarna SK, Layton C, Bancroft JD, editors. 2019. Transmission electron microscopy. Bancroft's Theory and Practice of Histological Techniques. 8th ed. Elsevier;London: p. 434–75.29. Beauchamp C, Fridovich I. 1971; Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 44:276–87. DOI: 10.1016/0003-2697(71)90370-8. PMID: 4943714.
Article30. Sinha AK. 1972; Colorimetric assay of catalase. Anal Biochem. 47:389–94. DOI: 10.1016/0003-2697(72)90132-7. PMID: 4556490.
Article31. Ohkawa H, Ohishi N, Yagi K. 1979; Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–8. DOI: 10.1016/0003-2697(79)90738-3. PMID: 36810.
Article32. Rosner B. 2015. Fundamentals of biostatistics. 8th ed. Cengage Learning;Boston:33. Bilgin G, Kismet K, Kuru S, Kaya F, Senes M, Bayrakceken Y, Yumusak N, Celikkan FT, Erdemli E, Celemli OG, Sorkun K, Koca G. 2016; Ultrastructural investigation of the protective effects of propolis on bleomycin induced pulmonary fibrosis. Biotech Histochem. 91:195–203. DOI: 10.3109/10520295.2015.1123294. PMID: 26960158.
Article34. Dong SH, Liu YW, Wei F, Tan HZ, Han ZD. 2017; Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (BLM) via suppressing pro-fibrotic and inflammatory signaling pathways. Biomed Pharmacother. 89:1297–309. DOI: 10.1016/j.biopha.2017.03.005. PMID: 28320097.
Article35. Arezzini B, Vecchio D, Signorini C, Stringa B, Gardi C. 2018; F2-isoprostanes can mediate bleomycin-induced lung fibrosis. Free Radic Biol Med. 115:1–9. DOI: 10.1016/j.freeradbiomed.2017.11.007. PMID: 29129520.
Article36. Jang SS, Kim HG, Han JM, Lee JS, Choi MK, Huh GJ, Son CG. 2015; Modulation of radiation-induced alterations in oxidative stress and cytokine expression in lung tissue by Panax ginseng extract. Phytother Res. 29:201–9. DOI: 10.1002/ptr.5223. PMID: 25219493.37. Altintas N, Erboga M, Aktas C, Bilir B, Aydin M, Sengul A, Ates Z, Topcu B, Gurel A. 2016; Protective effect of infliximab, a tumor necrosis factor-alfa inhibitor, on bleomycin-induced lung fibrosis in rats. Inflammation. 39:65–78. DOI: 10.1007/s10753-015-0224-z. PMID: 26253295.
Article38. Tian SL, Yang Y, Liu XL, Xu QB. 2018; Emodin attenuates bleomycin-induced pulmonary fibrosis via anti-inflammatory and anti-oxidative activities in rats. Med Sci Monit. 24:1–10. DOI: 10.12659/MSM.905496. PMID: 29290631. PMCID: PMC5759514.
Article39. Tang H, Gao L, Mao J, He H, Liu J, Cai X, Lin H, Wu T. 2016; Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones. 21:239–49. DOI: 10.1007/s12192-015-0654-4. PMID: 26577463. PMCID: PMC4786523.
Article40. Mazzoli-Rocha F, Carvalho GM, Lanzetti M, Valença SS, Silva LF, Saldiva PH, Zin WA, Faffe DS. 2014; Respiratory toxicity of repeated exposure to particles produced by traffic and sugar cane burning. Respir Physiol Neurobiol. 191:106–13. DOI: 10.1016/j.resp.2013.11.004. PMID: 24280381.
Article41. Rago F, Melo EM, Kraemer L, Galvão I, Cassali GD, Santos RAS, Russo RC, Teixeira MM. 2019; Effect of preventive or therapeutic treatment with angiotensin 1-7 in a model of bleomycin-induced lung fibrosis in mice. J Leukoc Biol. 106:677–86. DOI: 10.1002/JLB.MA1218-490RR. PMID: 31256436.
Article42. Li L, Li D, Xu L, Zhao P, Deng Z, Mo X, Li P, Qi L, Li J, Gao J. 2015; Total extract of Yupingfeng attenuates bleomycin-induced pulmonary fibrosis in rats. Phytomedicine. 22:111–9. DOI: 10.1016/j.phymed.2014.10.011. PMID: 25636879.
Article43. Meng L, Zhang X, Wang H, Dong H, Gu X, Yu X, Liu Y. 2019; Yangyin Yiqi Mixture ameliorates Bleomycin-induced pulmonary fibrosis in rats through inhibiting TGF-β1/Smad pathway and epithelial to mesenchymal transition. Evid Based Complement Alternat Med. 2019:2710509. DOI: 10.1155/2019/2710509. PMID: 30719057. PMCID: PMC6335662.
Article44. Chen F, Wang PL, Fan XS, Yu JH, Zhu Y, Zhu ZH. 2016; Effect of Renshen Pingfei Decoction, a traditional Chinese prescription, on IPF induced by Bleomycin in rats and regulation of TGF-β1/Smad3. J Ethnopharmacol. 186:289–97. DOI: 10.1016/j.jep.2016.03.051. PMID: 27013092.
Article45. Tawfik MK, Makary S. 2017; 5-HT7 receptor antagonism (SB-269970) attenuates bleomycin-induced pulmonary fibrosis in rats via downregulating oxidative burden and inflammatory cascades and ameliorating collagen deposition: comparison to terguride. Eur J Pharmacol. 814:114–23. DOI: 10.1016/j.ejphar.2017.08.014. PMID: 28821451.
Article46. Liu J, Han Z, Han Z, He Z. 2016; Mesenchymal stem cell-conditioned media suppresses inflammation-associated overproliferation of pulmonary artery smooth muscle cells in a rat model of pulmonary hypertension. Exp Ther Med. 11:467–75. DOI: 10.3892/etm.2015.2953. PMID: 26893632. PMCID: PMC4734026.
Article47. Zaafan MA, Zaki HF, El-Brairy AI, Kenawy SA. 2016; Pyrrolidinedithiocarbamate attenuates bleomycin-induced pulmonary fibrosis in rats: modulation of oxidative stress, fibrosis, and inflammatory parameters. Exp Lung Res. 42:408–16. DOI: 10.1080/01902148.2016.1244578. PMID: 27797599.
Article48. Zuo WL, Zhao JM, Huang JX, Zhou W, Lei ZH, Huang YM, Huang YF, Li HG. 2017; Effect of bosentan is correlated with MMP-9/TIMP-1 ratio in bleomycin-induced pulmonary fibrosis. Biomed Rep. 6:201–5. DOI: 10.3892/br.2016.832. PMID: 28357073. PMCID: PMC5351104.
Article49. Goto K, Imaoka M, Goto M, Kikuchi I, Suzuki T, Jindo T, Takasaki W. 2013; Effect of body-weight loading onto the articular cartilage on the occurrence of quinolone-induced chondrotoxicity in juvenile rats. Toxicol Lett. 216:124–9. DOI: 10.1016/j.toxlet.2012.11.017. PMID: 23201441.
Article50. Li Y, Wang S, Wang Y, Zhou C, Chen G, Shen W, Li C, Lin W, Lin S, Huang H, Liu P, Shen X. 2013; Inhibitory effect of the antimalarial agent artesunate on collagen-induced arthritis in rats through nuclear factor kappa B and mitogen-activated protein kinase signaling pathway. Transl Res. 161:89–98. DOI: 10.1016/j.trsl.2012.06.001. PMID: 22749778.
Article51. Li Q, Mao M, Qiu Y, Liu G, Sheng T, Yu X, Wang S, Zhu D. 2016; Key role of ROS in the process of 15-lipoxygenase/15-hydroxyeicosatetraenoiccid-induced pulmonary vascular remodeling in hypoxia pulmonary hypertension. PLoS One. 11:e0149164. DOI: 10.1371/journal.pone.0149164. PMID: 26871724. PMCID: PMC4752324.
Article52. Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L. 2018; Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol. 175:1279–92. DOI: 10.1111/bph.13828. PMID: 28430357. PMCID: PMC5867026.
Article53. Verma R, Kushwah L, Gohel D, Patel M, Marvania T, Balakrishnan S. 2013; Evaluating the ameliorative potential of quercetin against the bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med. 2013:921724. DOI: 10.1155/2013/921724. PMID: 24396596. PMCID: PMC3875129.
Article54. Sabry M, Taha HH, Mohamed AO, Thabet K, Hasan AA, Abdel-Razik ARH, Elsadek BEM. 2020; TLR4/NF-KB signaling pathway is a key pathogenic event of lung injury in bleomycin-induced pulmonary fibrosis in a mouse model. Az J Pharm Sci. 61:92–103. DOI: 10.21608/ajps.2020.86019.
Article55. Kato S, Inui N, Hakamata A, Suzuki Y, Enomoto N, Fujisawa T, Nakamura Y, Watanabe H, Suda T. 2018; Changes in pulmonary endothelial cell properties during bleomycin-induced pulmonary fibrosis. Respir Res. 19:127. DOI: 10.1186/s12931-018-0831-y. PMID: 29940932. PMCID: PMC6019800.
Article56. Kim MS, Kim SH, Jeon D, Kim HY, Lee K. 2018; Changes in expression of cytokines in polyhexamethylene guanidine-induced lung fibrosis in mice: Comparison of bleomycin-induced lung fibrosis. Toxicology. 393:185–92. DOI: 10.1016/j.tox.2017.11.017. PMID: 29175452.
Article57. Fanny M, Nascimento M, Baron L, Schricke C, Maillet I, Akbal M, Riteau N, Le Bert M, Quesniaux V, Ryffel B, Gombault A, Même S, Même W, Couillin I. 2018; The IL-33 receptor ST2 regulates pulmonary inflammation and fibrosis to bleomycin. Front Immunol. 9:1476. DOI: 10.3389/fimmu.2018.01476. PMID: 29988569. PMCID: PMC6026799.
Article58. Zhao L, Mu B, Zhou R, Cheng Y, Huang C. 2019; Iguratimod ameliorates bleomycin-induced alveolar inflammation and pulmonary fibrosis in mice by suppressing expression of matrix metalloproteinase-9. Int J Rheum Dis. 22:686–94. DOI: 10.1111/1756-185X.13463. PMID: 30666825.
Article59. Comeglio P, Filippi S, Sarchielli E, Morelli A, Cellai I, Corno C, Pini A, Adorini L, Vannelli GB, Maggi M, Vignozzi L. 2019; Therapeutic effects of obeticholic acid (OCA) treatment in a bleomycin-induced pulmonary fibrosis rat model. J Endocrinol Invest. 42:283–94. DOI: 10.1007/s40618-018-0913-1. PMID: 29923060.
Article60. Shamskhou EA, Kratochvil MJ, Orcholski ME, Nagy N, Kaber G, Steen E, Balaji S, Yuan K, Keswani S, Danielson B, Gao M, Medina C, Nathan A, Chakraborty A, Bollyky PL, De Jesus Perez VA. 2019; Hydrogel-based delivery of Il-10 improves treatment of bleomycin-induced lung fibrosis in mice. Biomaterials. 203:52–62. DOI: 10.1016/j.biomaterials.2019.02.017. PMID: 30852423. PMCID: PMC6430662.
Article61. Okamoto A, Nojiri T, Konishi K, Tokudome T, Miura K, Hosoda H, Hino J, Miyazato M, Kyomoto Y, Asai K, Hirata K, Kangawa K. 2017; Atrial natriuretic peptide protects against bleomycin-induced pulmonary fibrosis via vascular endothelial cells in mice : ANP for pulmonary fibrosis. Respir Res. 18:1. DOI: 10.1186/s12931-016-0492-7. PMID: 28049526. PMCID: PMC5210263.
Article62. Kabel AM, Omar MS, Elmaaboud MAA. 2016; Amelioration of bleomycin-induced lung fibrosis in rats by valproic acid and butyrate: Role of nuclear factor kappa-B, proinflammatory cytokines and oxidative stress. Int Immunopharmacol. 39:335–42. DOI: 10.1016/j.intimp.2016.08.008. PMID: 27526269.
Article63. Mulero MC, Wang VY, Huxford T, Ghosh G. 2019; Genome reading by the NF-κB transcription factors. Nucleic Acids Res. 47:9967–89. DOI: 10.1093/nar/gkz739. PMID: 31501881. PMCID: PMC6821244.
Article64. Serasanambati M, Chilakapati SR. 2016; Function of nuclear factor kappa B (NF-kB) in human diseases- a review. South Indian J Biol Sci. 2:368–87. DOI: 10.22205/sijbs/2016/v2/i4/103443.65. Tilborghs S, Corthouts J, Verhoeven Y, Arias D, Rolfo C, Trinh XB, van Dam PA. 2017; The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit Rev Oncol Hematol. 120:141–50. DOI: 10.1016/j.critrevonc.2017.11.001. PMID: 29198328.
Article66. Qin X, Yan M, Wang X, Xu Q, Wang X, Zhu X, Shi J, Li Z, Zhang J, Chen W. 2018; Cancer-associated fibroblast-derived IL-6 promotes head and neck cancer progression via the Osteopontin-NF-kappa B signaling pathway. Theranostics. 8:921–40. DOI: 10.7150/thno.22182. PMID: 29463991. PMCID: PMC5817102.
Article67. Wu J, Ding J, Yang J, Guo X, Zheng Y. 2018; MicroRNA roles in the nuclear factor kappa B signaling pathway in cancer. Front Immunol. 9:546. DOI: 10.3389/fimmu.2018.00546. PMID: 29616037. PMCID: PMC5868594.
Article68. Alvira CM. 2014; Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions. Birth Defects Res A Clin Mol Teratol. 100:202–16. DOI: 10.1002/bdra.23233. PMID: 24639404. PMCID: PMC4158903.
Article69. Hou J, Ma T, Cao H, Chen Y, Wang C, Chen X, Xiang Z, Han X. 2018; TNF-α-induced NF-κB activation promotes myofibroblast differentiation of LR-MSCs and exacerbates bleomycin-induced pulmonary fibrosis. J Cell Physiol. 233:2409–19. DOI: 10.1002/jcp.26112. PMID: 28731277.
Article70. Taooka Y. 2017; Neutrophil-mediating lung diseases and integrin α subfamily, α4 and α9. J Respir Res. 3:95–7. DOI: 10.17554/j.issn.2412-2424.2017.03.25.
Article71. Li G, Jin F, Du J, He Q, Yang B, Luo P. 2019; Macrophage-secreted TSLP and MMP9 promote bleomycin-induced pulmonary fibrosis. Toxicol Appl Pharmacol. 366:10–6. DOI: 10.1016/j.taap.2019.01.011. PMID: 30653976.
Article72. Tamò L, Simillion C, Hibaoui Y, Feki A, Gugger M, Prasse A, Jäger B, Goldmann T, Geiser T, Gazdhar A. 2018; Gene network analysis of interstitial macrophages after treatment with induced pluripotent stem cells secretome (iPSC-cm) in the bleomycin injured rat lung. Stem Cell Rev Rep. 14:412–24. DOI: 10.1007/s12015-017-9790-9. PMID: 29256173. PMCID: PMC5960485.
Article73. Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. 2015; Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Biol Interact. 237:151–65. DOI: 10.1016/j.cbi.2015.06.019. PMID: 26093215.
Article74. Zhao Q, Wu J, Lin Z, Hua Q, Zhang W, Ye L, Wu G, Du J, Xia J, Chu M, Hu X. 2016; Resolvin D1 alleviates the lung ischemia reperfusion injury via complement, immunoglobulin, TLR4, and inflammatory factors in rats. Inflammation. 39:1319–33. DOI: 10.1007/s10753-016-0364-9. PMID: 27145782. PMCID: PMC4951504.
Article75. Zhou Z, Kandhare AD, Kandhare AA, Bodhankar SL. 2019; Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-beta1/Smad3/AMPK and IkappaBalpha/NF-kappaB pathways. EXCLI J. 18:723–45. DOI: 10.17179/excli2019-1094. PMID: 31611754. PMCID: PMC6785776.76. Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K, Lee I. 2012; Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 1817:598–609. DOI: 10.1016/j.bbabio.2011.07.001. PMID: 21771582. PMCID: PMC3229836.
Article77. Liu X, Chen Z. 2017; The pathophysiological role of mitochondrial oxidative stress in lung diseases. J Transl Med. 15:207. DOI: 10.1186/s12967-017-1306-5. PMID: 29029603. PMCID: PMC5640915.
Article78. Burman A, Tanjore H, Blackwell TS. 2018; Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 68-69:355–65. DOI: 10.1016/j.matbio.2018.03.015. PMID: 29567124. PMCID: PMC6392005.
Article79. Arafa MH, Mohamed DA, Atteia HH. 2015; Ameliorative effect of N-acetyl cysteine on alpha-cypermethrin-induced pulmonary toxicity in male rats. Environ Toxicol. 30:26–43. DOI: 10.1002/tox.21891. PMID: 23900960.
Article80. El-Khouly D, El-Bakly WM, Awad AS, El-Mesallamy HO, El-Demerdash E. 2012; Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor Kappa-B in rats. Toxicology. 302:106–13. DOI: 10.1016/j.tox.2012.09.001. PMID: 22982510.
Article81. Bahri S, Ben Ali R, Gasmi K, Mlika M, Fazaa S, Ksouri R, Serairi R, Jameleddine S, Shlyonsky V. 2017; Prophylactic and curative effect of rosemary leaves extract in a bleomycin model of pulmonary fibrosis. Pharm Biol. 55:462–71. DOI: 10.1080/13880209.2016.1247881. PMID: 28093019. PMCID: PMC6130597.
Article82. Gao Y, Yao LF, Zhao Y, Wei LM, Guo P, Yu M, Cao B, Li T, Chen H, Zou ZM. 2016; The Chinese herbal medicine formula mKG suppresses pulmonary fibrosis of mice induced by bleomycin. Int J Mol Sci. 17:238. DOI: 10.3390/ijms17020238. PMID: 26891294. PMCID: PMC4783969.
Article83. Zhou Y, Liao S, Zhang Z, Wang B, Wan L. 2016; Astragalus injection attenuates bleomycin-induced pulmonary fibrosis via down-regulating Jagged1/Notch1 in lungs. J Pharm Pharmacol. 68:389–96. DOI: 10.1111/jphp.12518. PMID: 26817817.
Article84. Saba E, Jeon BR, Jeong DH, Lee K, Goo YK, Kwak D, Kim S, Roh SS, Kim SD, Nah SY, Rhee MH. 2015; A novel Korean red ginseng compound gintonin inhibited inflammation by MAPK and NF-κB pathways and recovered the levels of mir-34a and mir-93 in RAW 264.7 cells. Evid Based Complement Alternat Med. 2015:624132. DOI: 10.1155/2015/624132. PMID: 26579204. PMCID: PMC4633694.
Article85. Ahn S, Singh P, Castro-Aceituno V, Yesmin Simu S, Kim YJ, Mathiyalagan R, Yang DC. 2017; Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory - mediators production via blockade of NF-κB activation in macrophages. Artif Cells Nanomed Biotechnol. 45:270–6. DOI: 10.1080/21691401.2016.1228661. PMID: 27611566.
Article86. Ren J, Wang LX, Ji XC, Zhou JY, Zhang MY. 2013; Ultrastructural observation on pulmonary fibrosis in E9 rats treated with compound Carapax trionycis formula. Asian Pac J Trop Med. 6:153–5. DOI: 10.1016/S1995-7645(13)60013-9. PMID: 23339920.
Article87. Im K, Kim J, Min H. 2016; Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection. J Ginseng Res. 40:309–14. DOI: 10.1016/j.jgr.2015.09.002. PMID: 27746682. PMCID: PMC5052424.
Article88. Hsieh YH, Deng JS, Chang YS, Huang GJ. 2018; Ginsenoside Rh2 ameliorates lipopolysaccharide-induced acute lung injury by regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 signaling pathways in mice. Nutrients. 10:1208. DOI: 10.20944/preprints201807.0426.v1. PMID: 30200495. PMCID: PMC6163254.89. Hafez MM, Hamed SS, El-Khadragy MF, Hassan ZK, Al Rejaie SS, Sayed-Ahmed MM, Al-Harbi NO, Al-Hosaini KA, Al-Harbi MM, Alhoshani AR, Al-Shabanah OA, Alsharari SD. 2017; Effect of ginseng extract on the TGF-β1 signaling pathway in CCl4-induced liver fibrosis in rats. BMC Complement Altern Med. 17:45. DOI: 10.1186/s12906-016-1507-0. PMID: 28086769. PMCID: PMC5237131.
Article90. Mansour HH. 2013; Protective effect of ginseng against gamma-irradiation-induced oxidative stress and endothelial dysfunction in rats. EXCLI J. 12:766–77. PMID: 26622217. PMCID: PMC4662181.91. Zhang XH, Xu XX, Xu T. 2015; Ginsenoside Ro suppresses interleukin-1β-induced apoptosis and inflammation in rat chondrocytes by inhibiting NF-κB. Chin J Nat Med. 13:283–9. DOI: 10.1016/S1875-5364(15)30015-7. PMID: 25908625.
Article92. Rastogi V, Santiago-Moreno J, Doré S. 2015; Ginseng: a promising neuroprotective strategy in stroke. Front Cell Neurosci. 8:457. DOI: 10.3389/fncel.2014.00457. PMID: 25653588. PMCID: PMC4299449.
Article93. Duguran DR, Lopez MJC, Valenzuela MTCE, Ples MB, Vitor II RJS. 2018; Protective potential of ginseng and silymarin on the liver and kidney of ethanol-treated mice (Mus musculus). Natl J Physiol Pharm Pharmacol. 8:969–76. DOI: 10.5455/njppp.2018.8.0208104032018.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amelioration of Bleomycin-induced Pulmonary Fibrosis of Rats by an Aldose Reductase Inhibitor, Epalrestat
- The Effects of Chronic Intermittent Hypoxia in Bleomycin-Induced Lung Injury on Pulmonary Fibrosis via Regulating the NF-κB/Nrf2 Signaling Pathway
- A review of current studies on cellular and molecular mechanisms underlying pulmonary fibrosis induced by chemicals
- Early and Late Changes of MMP-2 and MMP-9 in Bleomycin-Induced Pulmonary Fibrosis
- A case of severe bleomycin-induced pulmonary fibrosis: reversal with high dose prednisolone