Diabetes Metab J.
2021 Nov;45(6):868-879. 10.4093/dmj.2020.0149.
Associations of Plasma Glucagon Levels with Estimated Glomerular Filtration Rate, Albuminuria and Diabetic Kidney Disease in Patients with Type 2 Diabetes Mellitus
- Affiliations
-
- 1Department of General Medicine, First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Nephrology, Affiliated Hospital 2 of Nantong University, and First People’s Hospital of Nantong City, Nantong, China
- 3Department of Endocrinology, Affiliated Hospital 2 of Nantong University, and First People’s Hospital of Nantong City, Nantong, China
- 4Medical Research Center, Affiliated Hospital 2 of Nantong University, and First People’s Hospital of Nantong City, Nantong, China
- 5Department of Clinical Laboratory, Affiliated Hospital 2 of Nantong University, and First People’s Hospital of Nantong City, Nantong, China
- KMID: 2522726
- DOI: http://doi.org/10.4093/dmj.2020.0149
Abstract
- Background
Type 2 diabetes mellitus (T2DM) is characterized by elevated fasting glucagon and impaired suppression of postprandial glucagon secretion, which may participate in diabetic complications. Therefore, we investigated the associations of plasma glucagon with estimated glomerular filtration rate (eGFR), albuminuria and diabetic kidney disease (DKD) in T2DM patients.
Methods
Fasting glucagon and postchallenge glucagon (assessed by area under the glucagon curve [AUCgla]) levels were determined during oral glucose tolerance tests. Patients with an eGFR <60 mL/min/1.73 m2 and/or a urinary albumin-to-creatinine ratio (UACR) ≥30 mg/g who presented with diabetic retinopathy were identified as having DKD.
Results
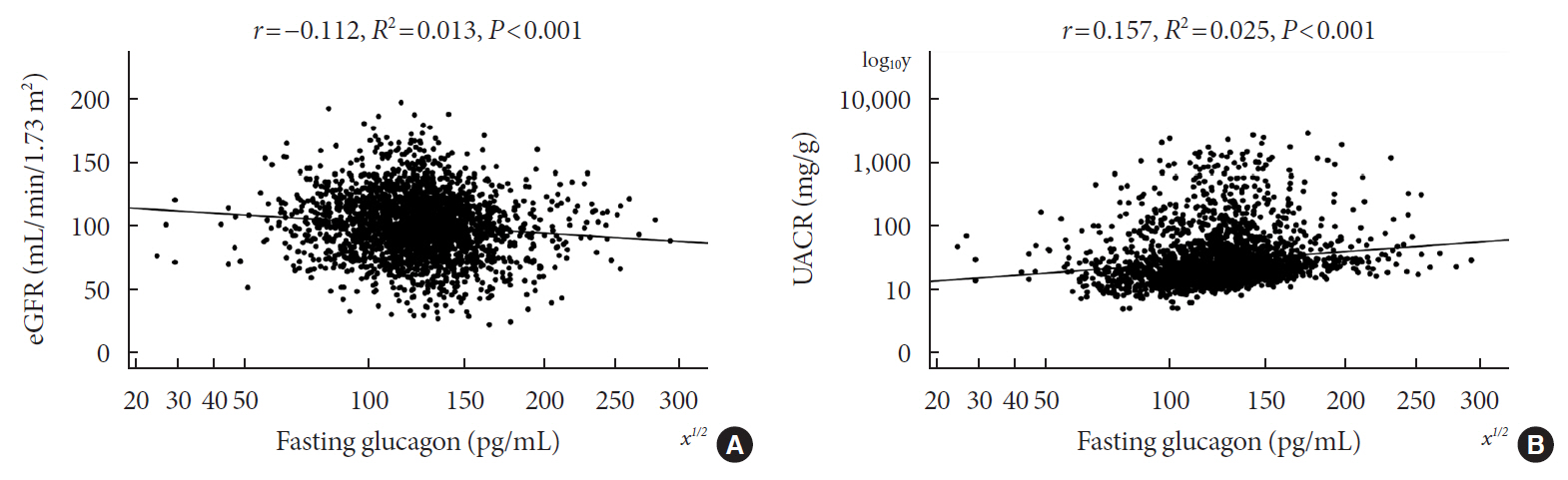
Of the 2,436 recruited patients, fasting glucagon was correlated with eGFR and UACR (r=–0.112 and r=0.157, respectively; P<0.001), and AUCgla was also correlated with eGFR and UACR (r=–0.267 and r=0.234, respectively; P<0.001). Moreover, 31.7% (n=771) presented with DKD; the prevalence of DKD was 27.3%, 27.6%, 32.5%, and 39.2% in the first (Q1), second (Q2), third (Q3), and fourth quartile (Q4) of fasting glucagon, respectively; and the corresponding prevalence for AUCgla was 25.9%, 22.7%, 33.7%, and 44.4%, respectively. Furthermore, after adjusting for other clinical covariates, the adjusted odds ratios (ORs; 95% confidence intervals) for DKD in Q2, Q3, and Q4 versus Q1 of fasting glucagon were 0.946 (0.697 to 1.284), 1.209 (0.895 to 1.634), and 1.521 (1.129 to 2.049), respectively; the corresponding ORs of AUCgla were 0.825 (0.611 to 1.114), 1.323 (0.989 to 1.769), and 2.066 (1.546 to 2.760), respectively. Additionally, when we restricted our analysis in patients with glycosylated hemoglobin <7.0% (n=471), we found fasting glucagon and AUCgla were still independently associated with DKD.
Conclusion
Both increased fasting and postchallenge glucagon levels were independently associated with DKD in T2DM patients.
Figure
Reference
-
1. Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016; 316:602–10.
Article2. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018; 25:121–32.
Article3. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019; 62:3–16.
Article4. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012; 380:1662–73.
Article5. Mohammedi K, Woodward M, Marre M, Colagiuri S, Cooper M, Harrap S, et al. Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes. Cardiovasc Diabetol. 2017; 16:95.
Article6. Wewer Albrechtsen NJ, Faerch K, Jensen TM, Witte DR, Pedersen J, Mahendran Y, et al. Evidence of a liver-alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia. 2018; 61:671–80.
Article7. Knop FK, Vilsboll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia. 2007; 50:797–805.
Article8. Lund A, Bagger JI, Christensen M, Grondahl M, van Hall G, Holst JJ, et al. Higher endogenous glucose production during OGTT vs isoglycemic intravenous glucose infusion. J Clin Endocrinol Metab. 2016; 101:4377–84.
Article9. Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sorensen M, Suppli MP, Janah L, et al. The liver-α-cell axis and type 2 diabetes. Endocr Rev. 2019; 40:1353–66.
Article10. Holst JJ, Holland W, Gromada J, Lee Y, Unger RH, Yan H, et al. Insulin and glucagon: partners for life. Endocrinology. 2017; 158:696–701.
Article11. Li XC, Liao TD, Zhuo JL. Long-term hyperglucagonaemia induces early metabolic and renal phenotypes of type 2 diabetes in mice. Clin Sci (Lond). 2008; 114:591–601.12. Wang X, Yang J, Chang B, Shan C, Xu Y, Zheng M, et al. Glucagon secretion is increased in patients with type 2 diabetic nephropathy. J Diabetes Complications. 2016; 30:488–93.
Article13. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011; 34(Suppl 1):S62–9.14. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012; 367:20–9.
Article15. De Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011; 305:2532–9.
Article16. Gosmanov AR, Wall BM, Gosmanova EO. Diagnosis and treatment of diabetic kidney disease. Am J Med Sci. 2014; 347:406–13.
Article17. Radaelli T, Farrell KA, Huston-Presley L, Amini SB, Kirwan JP, McIntyre HD, et al. Estimates of insulin sensitivity using glucose and C-Peptide from the hyperglycemia and adverse pregnancy outcome glucose tolerance test. Diabetes Care. 2010; 33:490–4.
Article18. Zhao L, Ma J, Wang S, Xie Y. Relationship between β-cell function, metabolic control, and microvascular complications in type 2 diabetes mellitus. Diabetes Technol Ther. 2015; 17:29–34.
Article19. Su JB, Wu YY, Xu F, Wang X, Cai HL, Zhao LH, et al. Serum complement C3 and islet β-cell function in patients with type 2 diabetes: a 4.6-year prospective follow-up study. Endocrine. 2020; 67:321–30.
Article20. Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014; 63(2 Suppl 2):S39–62.
Article21. Guo K, Zhang L, Zhao F, Lu J, Pan P, Yu H, et al. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: cross-sectional study. J Diabetes Complications. 2016; 30:803–10.
Article22. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017; 12:2032–45.23. Alicic RZ, Johnson EJ, Tuttle KR. Inflammatory mechanisms as new biomarkers and therapeutic targets for diabetic kidney disease. Adv Chronic Kidney Dis. 2018; 25:181–91.
Article24. Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol. 2017; 312:F716–31.
Article25. Looker HC, Mauer M, Nelson RG. Role of kidney biopsies for biomarker discovery in diabetic kidney disease. Adv Chronic Kidney Dis. 2018; 25:192–201.
Article26. Sharma D, Bhattacharya P, Kalia K, Tiwari V. Diabetic nephropathy: new insights into established therapeutic paradigms and novel molecular targets. Diabetes Res Clin Pract. 2017; 128:91–108.
Article27. Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 2018; 71:884–95.
Article28. Janah L, Kjeldsen S, Galsgaard KD, Winther-Sorensen M, Stojanovska E, Pedersen J, et al. Glucagon receptor signaling and glucagon resistance. Int J Mol Sci. 2019; 20:3314.
Article29. Takahashi N, Chujo D, Kajio H, Ueki K. Contribution of pancreatic α-cell function to insulin sensitivity and glycemic variability in patients with type 1 diabetes. J Diabetes Investig. 2019; 10:690–8.30. Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007; 28:253–83.31. Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012; 153:1039–48.
Article32. Kilpatrick ES, Rigby AS, Atkin SL. Mean blood glucose compared with HbA1c in the prediction of cardiovascular disease in patients with type 1 diabetes. Diabetologia. 2008; 51:365–71.
Article33. Takao T, Matsuyama Y, Yanagisawa H, Kikuchi M, Kawazu S. Association between HbA1c variability and mortality in patients with type 2 diabetes. J Diabetes Complications. 2014; 28:494–9.
Article34. Ali S, Ussher JR, Baggio LL, Kabir MG, Charron MJ, Ilkayeva O, et al. Cardiomyocyte glucagon receptor signaling modulates outcomes in mice with experimental myocardial infarction. Mol Metab. 2014; 4:132–43.
Article35. Gao C, Ren SV, Yu J, Baal U, Thai D, Lu J, et al. Glucagon receptor antagonism ameliorates progression of heart failure. JACC Basic Transl Sci. 2019; 4:161–72.
Article36. Sharma AX, Quittner-Strom EB, Lee Y, Johnson JA, Martin SA, Yu X, et al. Glucagon receptor antagonism improves glucose metabolism and cardiac function by promoting AMP-mediated protein kinase in diabetic mice. Cell Rep. 2018; 22:1760–73.
Article37. Wewer Albrechtsen NJ, Junker AE, Christensen M, Haedersdal S, Wibrand F, Lund AM, et al. Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Am J Physiol Gastrointest Liver Physiol. 2018; 314:G91–6.
Article38. Kim JA, Choi HJ, Kwon YK, Ryu DH, Kwon TH, Hwang GS. 1H NMR-based metabolite profiling of plasma in a rat model of chronic kidney disease. PLoS One. 2014; 9:e85445.
Article39. Hanifa MA, Skott M, Maltesen RG, Rasmussen BS, Nielsen S, Frokiaer J, et al. Tissue, urine and blood metabolite signatures of chronic kidney disease in the 5/6 nephrectomy rat model. Metabolomics. 2019; 15:112.
Article40. Ahloulay M, Dechaux M, Laborde K, Bankir L. Influence of glucagon on GFR and on urea and electrolyte excretion: direct and indirect effects. Am J Physiol. 1995; 269(2 Pt 2):F225–35.
Article41. Liu JJ, Liu S, Gurung RL, Chan C, Ang K, Tang WE, et al. Relationship between fasting plasma glucagon level and renal function: a cross-sectional study in individuals with type 2 diabetes. J Endocr Soc. 2018; 3:273–83.42. Ying C, Zhou X, Chang Z, Ling H, Cheng X, Li W. Blood glucose fluctuation accelerates renal injury involved to inhibit the AKT signaling pathway in diabetic rats. Endocrine. 2016; 53:81–96.
Article43. Wang C, Song J, Ma Z, Yang W, Li C, Zhang X, et al. Fluctuation between fasting and 2-H postload glucose state is associated with chronic kidney disease in previously diagnosed type 2 diabetes patients with HbA1c ≥ 7%. PLoS One. 2014; 9:e102941.
Article44. Li XC, Zhuo JL. Targeting glucagon receptor signalling in treating metabolic syndrome and renal injury in type 2 diabetes: theory versus promise. Clin Sci (Lond). 2007; 113:183–93.45. Li XC, Carretero OA, Shao Y, Zhuo JL. Glucagon receptor-mediated extracellular signal-regulated kinase 1/2 phosphorylation in rat mesangial cells: role of protein kinase A and phospholipase C. Hypertension. 2006; 47:580–5.46. Li XC, Carretero OA, Zhuo JL. Cross-talk between angiotensin II and glucagon receptor signaling mediates phosphorylation of mitogen-activated protein kinases ERK 1/2 in rat glomerular mesangial cells. Biochem Pharmacol. 2006; 71:1711–9.
Article47. Bagger JI, Knop FK, Holst JJ, Vilsboll T. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab. 2011; 13:965–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening and Management of Diabetic Nephropathy
- 2021 Korean Diabetes Association Clinical Practice Guidelines for Diabetes: Diabetic Kidney Disease
- Soluble Dipeptidyl Peptidase-4 Levels Are Associated with Decreased Renal Function in Patients with Type 2 Diabetes Mellitus
- Clinical Significance of Decreased Glomerular Filtration Rate (GFR) without Albuminuria among Type 2 Diabetics (Korean Diabetes Journal 32(3):252-258, 2008)
- Evaluation of Plasma Neutrophil Gelatinase-Associated Lipocalin as a Biomarker for Tubular Damage in Diabetic Nephropathy