Int J Stem Cells.
2021 Nov;14(4):434-446. 10.15283/ijsc20118.
MiR-148a-3p Regulates the Invasion and Odontoblastic Differentiation of Human Dental Pulp Stem Cells via the Wnt1/β-Catenin Pathway
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Jingmen NO.1 People’s Hospital, Jingmen, China
- KMID: 2522562
- DOI: http://doi.org/10.15283/ijsc20118
Abstract
- Background and Objectives
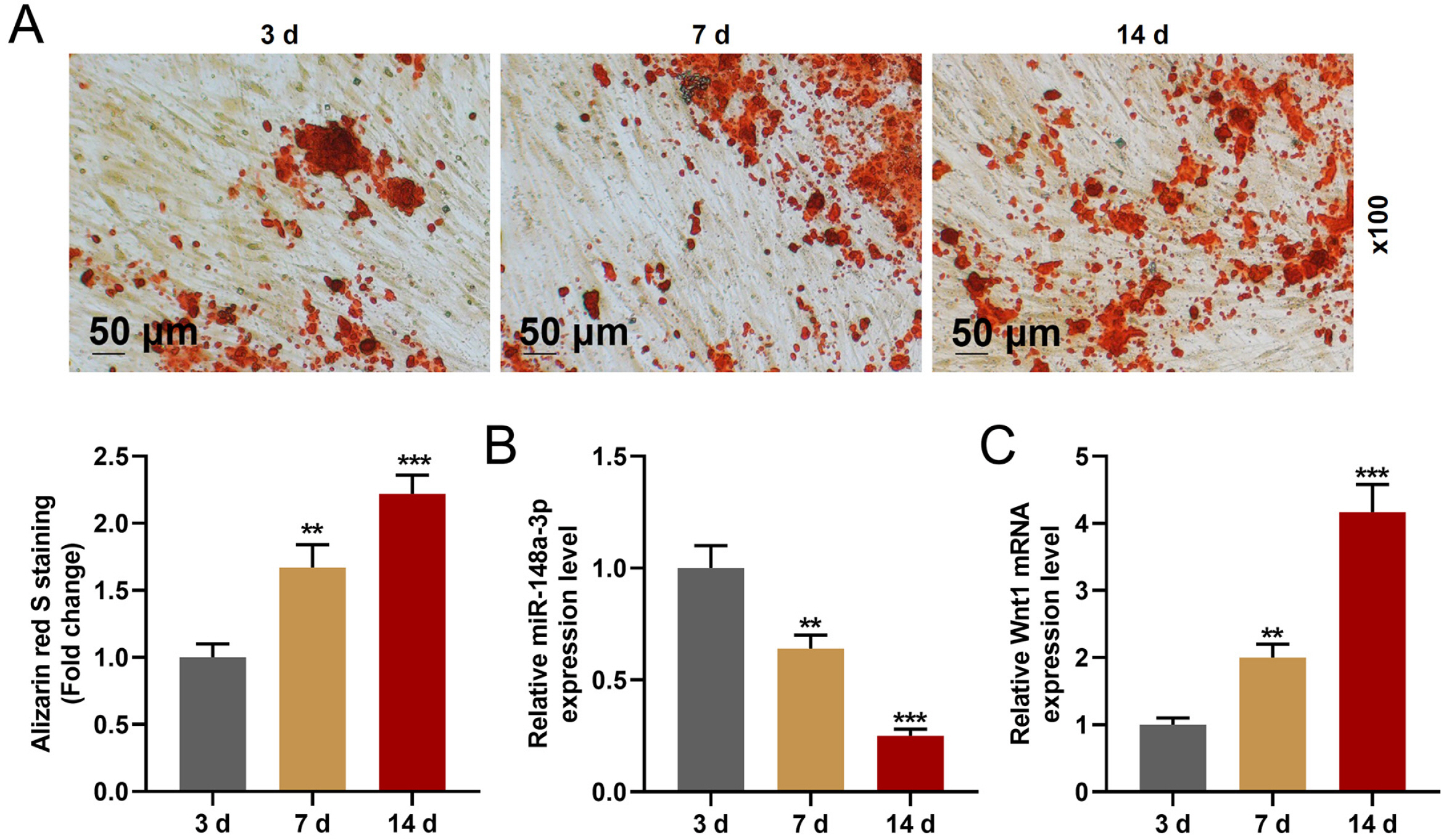
MiR-148a-3p has been reported to regulate the differentiation of marrow stromal cell osteoblast. In this study, whether miR-148a-3p regulated the odontoblastic differentiation of human dental pulp stem cells (hDPSCs) or not was explored.
Methods and Results
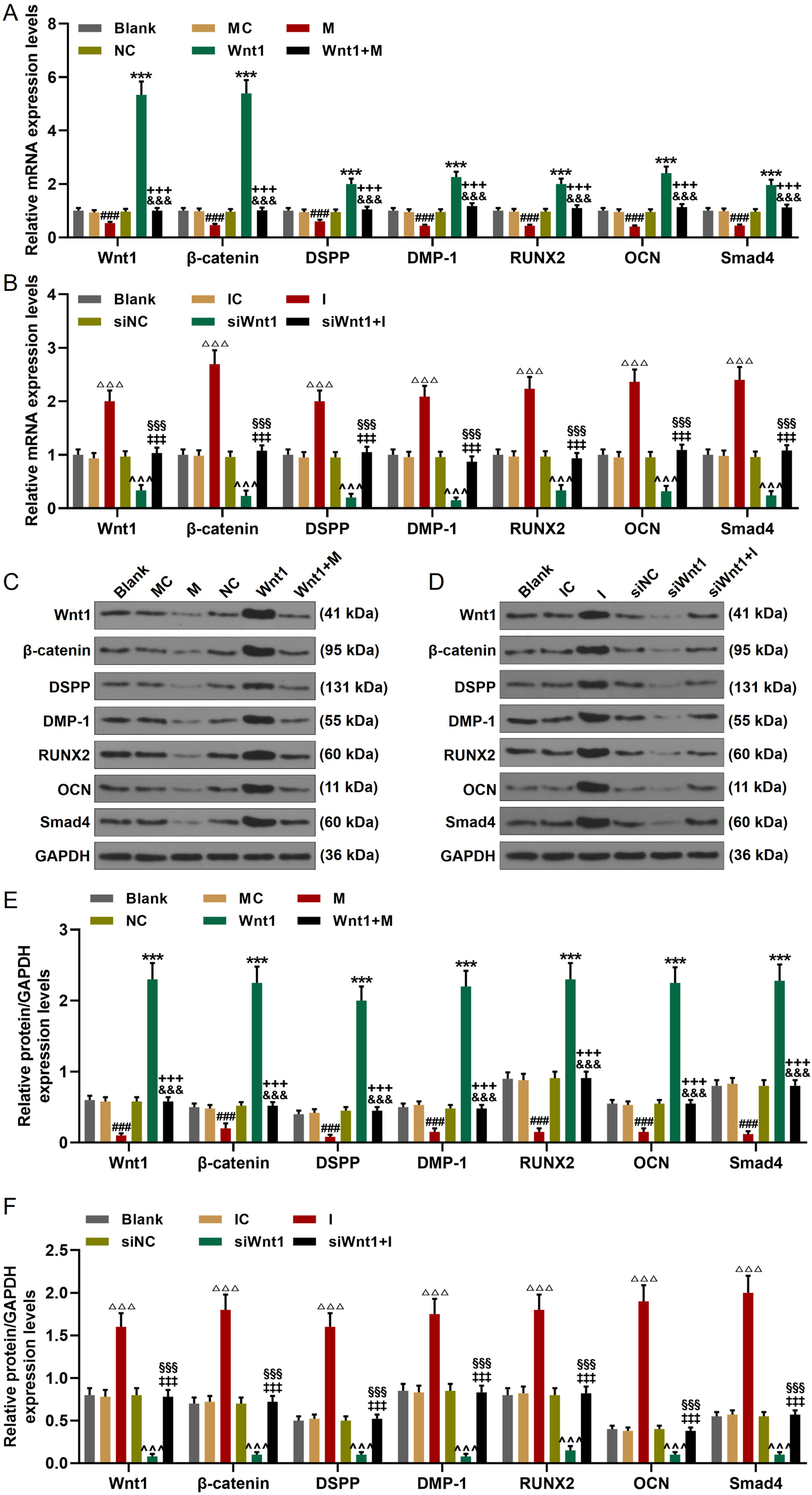
The hDPSCs were isolated and identified via flow cytometry. Targets of miR-148a-3p were identi-fied via bioinformatics and dual-luciferase reporter assay. After the cell was cultured in the odontogenic differentiation medium or infected, cell viability, invasion, and odontoblastic differentiation were detected via MTT, transwell, and Alizarin Red S staining, respectively. The miR-148a-3p, Wnt1, β-catenin, DSPP, DMP-1, RUNX2, OCN, and Smad4 expressions were determined by RT-qPCR and Western blot. The hDPSCs odontoblastic differentiation downregulated the miR-148a-3p expression and upregulated Wnt1 expression. Wnt1 was determined as the target for miR-148a-3p. MiR-148a-3p mimic and siWnt1 suppressed the cell viability, invasion, and odontoblastic differentiation of hDPSCs and inhibited the Wnt1, β-catenin, DSPP, DMP-1, RUNX2, OCN, and Smad4 expressions. In contrast, miR-148a-3p inhibitor and overexpressed Wnt1 promoted the cell viability, invasion, and odontoblastic differentiation of hDPSCs, and upregulated the Wnt1, β-catenin, DSPP, DMP-1, RUNX2, OCN, and Smad4 expressions. Also, miR-148a-3p mimic and inhibitor reversed the effects of Wnt1 overexpression and siWnt1.
Conclusions
MiR-148a-3p modulated the invasion and odontoblastic differentiation of hDPSCs through the Wnt1/β-catenin pathway.
Figure
Reference
-
References
1. Alsaeedi HA, Lam C, Koh AE, Teh SW, Mok PL, Higuchi A, Then KY, Bastion MC, Alzahrani B, Farhana A, Muthuvenkatachalam BS, Samrot AV, Swamy KB, Marraiki N, Elgorban AM, Subbiah SK. 2020; Looking into dental pulp stem cells in the therapy of photoreceptors and retinal degenerative disorders. J Photochem Photobiol B. 203:111727. DOI: 10.1016/j.jphotobiol.2019.111727. PMID: 31862637.
Article2. Lu X, Chen X, Xing J, Lian M, Huang D, Lu Y, Feng G, Feng X. 2019; miR-140-5p regulates the odontoblastic differentiation of dental pulp stem cells via the Wnt1/β-catenin signaling pathway. Stem Cell Res Ther. 10:226. DOI: 10.1186/s13287-019-1344-4. PMID: 31358066. PMCID: PMC6664499.
Article3. Mirhosseini M, Shiari R, Esmaeili Motlagh P, Farivar S. 2019; Cerebrospinal fluid and photobiomodulation effects on neural gene expression in dental pulp stem cells. J Lasers Med Sci. 10(Suppl 1):S30–S36. DOI: 10.15171/jlms.2019.S6. PMID: 32021670. PMCID: PMC6983870.
Article4. Wang W, Yuan C, Geng T, Liu Y, Zhu S, Zhang C, Liu Z, Wang P. 2020; EphrinB2 overexpression enhances osteogenic differentiation of dental pulp stem cells partially through ephrinB2-mediated reverse signaling. Stem Cell Res Ther. 11:40. DOI: 10.1186/s13287-019-1540-2. PMID: 31996240. PMCID: PMC6990579.
Article5. Qiao W, Li D, Shi Q, Wang H, Wang H, Guo J. 2020; miR-224-5p protects dental pulp stem cells from apoptosis by targeting Rac1. Exp Ther Med. 19:9–18. DOI: 10.3892/etm.2019.8213. PMID: 31897093. PMCID: PMC6923752.
Article6. Luke AM, Patnaik R, Kuriadom S, Abu-Fanas S, Mathew S, Shetty KP. 2020; Human dental pulp stem cells differentiation to neural cells, osteocytes and adipocytes-an in vitro study. Heliyon. 6:e03054. DOI: 10.1016/j.heliyon.2019.e03054. PMID: 32042932. PMCID: PMC7002807.
Article7. Bu NU, Lee HS, Lee BN, Hwang YC, Kim SY, Chang SW, Choi KK, Kim DS, Jang JH. 2020; In vitro characterization of dental pulp stem cells cultured in two microsphere-forming culture plates. J Clin Med. 9:242. DOI: 10.3390/jcm9010242. PMID: 31963371. PMCID: PMC7020027.
Article8. Kamel AHM, Kamal SM, AbuBakr N. 2020; Effect of smoking on the proliferation capacity and osteogenic potential of human dental pulp stem cells (DPSCs). Dent Med Probl. 57:19–24. DOI: 10.17219/dmp/113179. PMID: 31930782.
Article9. Kim Y, Park JY, Park HJ, Kim MK, Kim YI, Kim HJ, Bae SK, Bae MK. 2019; Pentraxin-3 modulates osteogenic/odontogenic differentiation and migration of human dental pulp stem cells. Int J Mol Sci. 20:5778. DOI: 10.3390/ijms20225778. PMID: 31744201. PMCID: PMC6887979.
Article10. Liang H, Li W, Yang H, Cao Y, Ge L, Shi R, Fan Z, Dong R, Zhang C. 2020; FAM96B inhibits the senescence of dental pulp stem cells. Cell Biol Int. 44:1193–1203. DOI: 10.1002/cbin.11319. PMID: 32039527.
Article11. Vishnoi A, Rani S. 2017; MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 1509:1–10. DOI: 10.1007/978-1-4939-6524-3_1. PMID: 27826912.
Article12. Xiaobing T, Qingyuan D. 2017; Characterization of microRNAs profiles of induced pluripotent stem cells reprogrammed from human dental pulp stem cells and stem cells from apical papilla. Hua Xi Kou Qiang Yi Xue Za Zhi. 35:269–274. Chinese. DOI: 10.7518/hxkq.2017.03.008. PMID: 28675011. PMCID: PMC7030426.13. Wang J, Zheng Y, Bai B, Song Y, Zheng K, Xiao J, Liang Y, Bao L, Zhou Q, Ji L, Feng X. 2020; MicroRNA-125a-3p participates in odontoblastic differentiation of dental pulp stem cells by targeting Fyn. Cytotechnology. 72:69–79. DOI: 10.1007/s10616-019-00358-7. PMID: 31953701. PMCID: PMC7002700.
Article14. Ke Z, Qiu Z, Xiao T, Zeng J, Zou L, Lin X, Hu X, Lin S, Lv H. 2019; Downregulation of miR-224-5p promotes migration and proliferation in human dental pulp stem cells. Biomed Res Int. 2019:4759060. DOI: 10.1155/2019/4759060. PMID: 31396530. PMCID: PMC6668552.
Article15. Cai SW, Han Y, Wang GP. 2018; miR-148a-3p exhaustion inhibits necrosis by regulating PTEN in acute pancreatitis. Int J Clin Exp Pathol. 11:5647–5657. PMID: 31949651. PMCID: PMC6963085.16. Pan L, Meng Q, Li H, Liang K, Li B. 2019; LINC00339 promotes cell proliferation, migration, and invasion of ovarian cancer cells via miR-148a-3p/ROCK1 axes. Biomed Pharmacother. 120:109423. DOI: 10.1016/j.biopha.2019.109423. PMID: 31550677.
Article17. Song B, Du J, Song DF, Ren JC, Feng Y. 2018; Dysregulation of NCAPG, KNL1, miR-148a-3p, miR-193b-3p, and miR-1179 may contribute to the progression of gastric cancer. Biol Res. 51:44. DOI: 10.1186/s40659-018-0192-5. PMID: 30390708. PMCID: PMC6215350.
Article18. Yuan H, Xu X, Feng X, Zhu E, Zhou J, Wang G, Tian L, Wang B. 2019; A novel long noncoding RNA PGC1β-OT1 regulates adipocyte and osteoblast differentiation through antagonizing miR-148a-3p. Cell Death Differ. 26:2029–2045. DOI: 10.1038/s41418-019-0296-7. PMID: 30728459. PMCID: PMC6748127.
Article19. Chen M, Yang Y, Zeng J, Deng Z, Wu B. 2020; circRNA expression profile in dental pulp stem cells during odontogenic differentiation. Stem Cells Int. 2020:5405931. DOI: 10.1155/2020/5405931. PMID: 32952566. PMCID: PMC7482017.
Article20. Labedz-Maslowska A, Bryniarska N, Kubiak A, Kaczmarzyk T, Sekula-Stryjewska M, Noga S, Boruczkowski D, Madeja Z, Zuba-Surma E. 2020; Multilineage differentiation potential of human dental pulp stem cells-impact of 3D and hypoxic environment on osteogenesis in vitro. Int J Mol Sci. 21:6172. DOI: 10.3390/ijms21176172. PMID: 32859105. PMCID: PMC7504399.
Article21. Gong Y, Yuan S, Sun J, Wang Y, Liu S, Guo R, Dong W, Li R. 2020; R-Spondin 2 induces odontogenic differentiation of dental pulp stem/progenitor cells via regulation of Wnt/β-catenin signaling. Front Physiol. 11:918. DOI: 10.3389/fphys.2020.00918. PMID: 32848860. PMCID: PMC7426510.
Article22. Kim YJ, Kim WJ, Bae SW, Yang SM, Park SY, Kim SM, Jung JY. 2021; Mineral trioxide aggregate-induced AMPK activation stimulates odontoblastic differentiation of human dental pulp cells. Int Endod J. 54:753–767. DOI: 10.1111/iej.13460. PMID: 33277707.
Article23. Li S, Lin C, Zhang J, Tao H, Liu H, Yuan G, Chen Z. 2018; Quaking promotes the odontoblastic differentiation of human dental pulp stem cells. J Cell Physiol. 233:7292–7304. DOI: 10.1002/jcp.26561. PMID: 29663385.
Article24. Feng G, Zhang J, Feng X, Wu S, Huang D, Hu J, Zhu S, Song D. 2016; Runx2 modified dental pulp stem cells (DPSCs) enhance new bone formation during rapid distraction osteogenesis (DO). Differentiation. 92:195–203. DOI: 10.1016/j.diff.2016.06.001. PMID: 27313006.
Article25. Xin BC, Wu QS, Jin S, Luo AH, Sun DG, Wang F. 2020; Berberine promotes osteogenic differentiation of human dental pulp stem cells through activating EGFR-MAPK-Runx2 pathways. Pathol Oncol Res. 26:1677–1685. DOI: 10.1007/s12253-019-00746-6. PMID: 31598896.
Article26. Friedrich M, Pracht K, Mashreghi MF, Jäck HM, Radbruch A, Seliger B. 2017; The role of the miR-148/-152 family in physiology and disease. Eur J Immunol. 47:2026–2038. DOI: 10.1002/eji.201747132. PMID: 28880997.
Article27. Zeng J, Zhu L, Liu J, Zhu T, Xie Z, Sun X, Zhang H. 2019; Metformin protects against oxidative stress injury induced by ischemia/reperfusion via regulation of the lncRNA-H19/miR-148a-3p/Rock2 axis. Oxid Med Cell Longev. 2019:8768327. DOI: 10.1155/2019/8768327. PMID: 31934270. PMCID: PMC6942897.
Article28. Dybos SA, Flatberg A, Halgunset J, Viset T, Rolfseng T, Kvam S, Skogseth H. 2018; Increased levels of serum miR-148a-3p are associated with prostate cancer. APMIS. 126:722–731. DOI: 10.1111/apm.12880. PMID: 30160020.
Article29. Liu F, Wang X, Yang Y, Hu R, Wang W, Wang Y. 2019; The suppressive effects of miR-508-5p on the odontogenic differentiation of human dental pulp stem cells by targeting glycoprotein non-metastatic melanomal protein B. Stem Cell Res Ther. 10:35. DOI: 10.1186/s13287-019-1146-8. PMID: 30670091. PMCID: PMC6341723.
Article30. Zhan FL, Liu XY, Wang XB. 2018; The role of MicroRNA-143-5p in the differentiation of dental pulp stem cells into odontoblasts by targeting Runx2 via the OPG/RANKL signaling pathway. J Cell Biochem. 119:536–546. DOI: 10.1002/jcb.26212. PMID: 28608628.
Article31. Wang W, Dong J, Wang M, Yao S, Tian X, Cui X, Fu S, Zhang S. 2018; miR-148a-3p suppresses epithelial ovarian cancer progression primarily by targeting c-Met. Oncol Lett. 15:6131–6136. DOI: 10.3892/ol.2018.8110. PMID: 29616095. PMCID: PMC5876423.
Article32. Song C, Yang J, Jiang R, Yang Z, Li H, Huang Y, Lan X, Lei C, Ma Y, Qi X, Chen H. 2019; miR-148a-3p regulates proliferation and apoptosis of bovine muscle cells by targeting KLF6. J Cell Physiol. [Epub ahead of print]. DOI: 10.1002/jcp.28232. PMID: 30793769.
Article33. Tang Y, Yang P, Zhu Y, Su Y. 2020; LncRNA TUG1 contributes to ESCC progression via regulating miR-148a-3p/MCL-1/Wnt/β-catenin axis in vitro. Thorac Cancer. 11:82–94. DOI: 10.1111/1759-7714.13236. PMID: 31742924. PMCID: PMC6938768.
Article34. Huang L, Ying H, Chen Z, Zhu YL, Gu Y, Hu L, Chen D, Zhong N. 2019; Down-regulation of DKK1 and Wnt1/β-catenin pathway by increased homeobox B7 resulted in cell differentiation suppression of intrauterine fetal growth retardation in human placenta. Placenta. 80:27–35. DOI: 10.1016/j.placenta.2019.03.001. PMID: 31103063.
Article35. Song P, Zheng JX, Liu JZ, Xu J, Wu LY, Liu C, Zhu Q, Wang Y. 2014; Effect of the Wnt1/β-catenin signalling pathway on human embryonic pulmonary fibroblasts. Mol Med Rep. 10:1030–1036. DOI: 10.3892/mmr.2014.2261. PMID: 24859686.
Article36. Zhang C, Hao Y, Sun Y, Liu P. 2019; Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. J Pharmacol Sci. 140:128–136. DOI: 10.1016/j.jphs.2019.03.005. PMID: 31257059.
Article37. Zhang JG, Shi Y, Hong DF, Song M, Huang D, Wang CY, Zhao G. 2015; MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway. Sci Rep. 5:8087. DOI: 10.1038/srep08087. PMID: 25627001. PMCID: PMC4310092.
Article38. Zhang X, Ning T, Wang H, Xu S, Yu H, Luo X, Hao C, Wu B, Ma D. 2019; Stathmin regulates the proliferation and odontoblastic/osteogenic differentiation of human dental pulp stem cells through Wnt/β-catenin signaling pathway. J Proteomics. 202:103364. DOI: 10.1016/j.jprot.2019.04.014. PMID: 31009804.
Article39. Ali M, Okamoto M, Komichi S, Watanabe M, Huang H, Takahashi Y, Hayashi M. 2019; Lithium-containing surface pre-reacted glass fillers enhance hDPSC functions and induce reparative dentin formation in a rat pulp capping model through activation of Wnt/β-catenin signaling. Acta Biomater. 96:594–604. DOI: 10.1016/j.actbio.2019.06.016. PMID: 31212112.
Article40. Scheller EL, Chang J, Wang CY. 2008; Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 87:126–130. DOI: 10.1177/154405910808700206. PMID: 18218837. PMCID: PMC2906770.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-27 Promotes Odontoblast Differentiation via Wnt1 Signaling
- MiR-29a-3p Inhibits Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells via Targeting FOXO3 and Repressing Wnt/β-Catenin Signaling in Steroid-Associated Osteonecrosis
- MiR-214 Regulates the Human Hair Follicle Stem Cell Proliferation and Differentiation by Targeting EZH2 and Wnt/β-Catenin Signaling Way In Vitro
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- miR-34a Inhibitor May Effectively Protect against Sevoflurane-Induced Hippocampal Apoptosis through the Wnt/β-Catenin Pathway by Targeting Wnt1