Obstet Gynecol Sci.
2021 Nov;64(6):506-516. 10.5468/ogs.21137.
Stage one endometrioid endometrial adenocarcinoma: is there a role of traditional hospital follow-up in the detection of cancer recurrence in women after treatment?
- Affiliations
-
- 1Department of Gynaecological Oncology, Epsom and St Helier University Hospitals & NHS Trust, St Helier Hospital, London, UK
- 2Department of Gynaecological Oncology, Queen Alexandra Hospital, Portsmouth, UK
- 3Barking, Havering and Redbridge University Hospitals & NHS Trust, Queen’s Hospital, London, UK
- KMID: 2522474
- DOI: http://doi.org/10.5468/ogs.21137
Abstract
Objective
To investigate the rate of asymptomatic recurrence of stage 1 endometrioid endometrial cancer and assess the role of routine hospital follow-up after treatment.
Methods
We performed a retrospective case-note review study of women who were diagnosed with stage 1 endometrioid endometrial adenocarcinoma at Queen’s Hospital, Romford, between January 2008 and December 2016.
Results
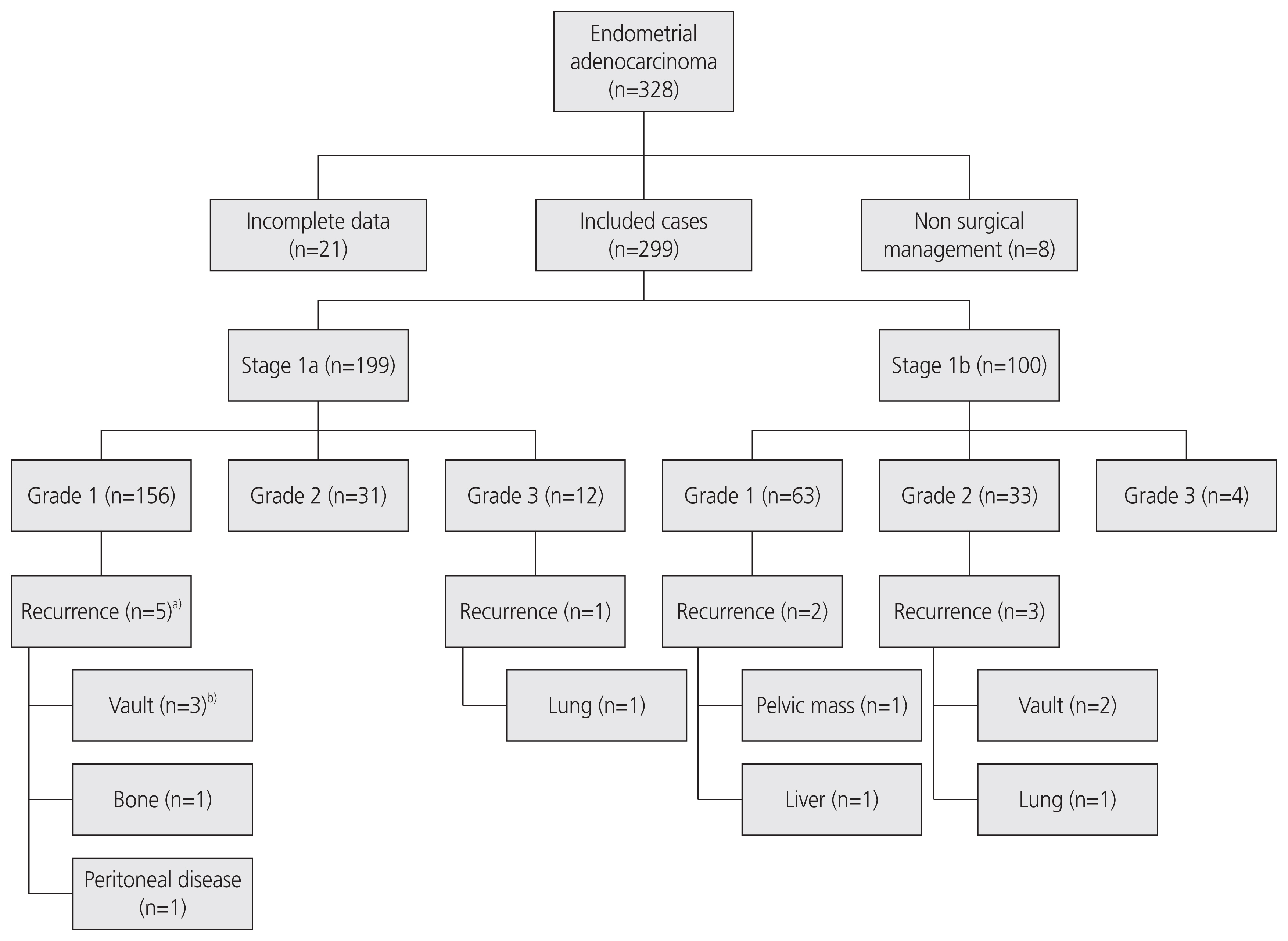
We included 299 patients with a median follow-up period of 44.4 months. All the patients underwent total hysterectomy and bilateral salpingo-oophorectomy. Adjuvant radiotherapy was offered to the patients subsequent to discussions in the multidisciplinary team meeting in accordance with the risk stratification criteria. There was no significant correlation between the risk factors and disease recurrence. In total, 11 patients presented with recurrent disease with original staging: 1a, n=6/199; and 1b, n=5/100. Four patients presented with vaginal bleeding due to vault recurrence and one patient with abdominal pain due to pelvic mass. Locoregional recurrence was an incidental finding in two other patients. Four patients presented with symptomatic distant metastases to the lung (n=2), liver (n=1), and bone (n=1). No asymptomatic recurrences were identified on routine follow-ups, despite several hospital appointments and clinical examinations. The recurrence rate for patients with stage 1a and 1b, grade 1, and grade 2 disease was 3.53%, and that for patients with stage 1a, grade 1, and grade 2 disease was 2.7%.
Conclusion
Routine clinical examinations have a low yield in finding recurrence in asymptomatic women and should be questioned for their value, considering the limited resources of the National Health Service (NHS). Larger studies are required to support a stratified follow-up, which will include telephone and patient-initiated follow-up.
Figure
Reference
-
References
1. Duncan ME, Seagroatt V, Goldacre MJ. Cancer of the body of the uterus: trends in mortality and incidence in England, 1985–2008. BJOG. 2012; 119:333–9.
Article2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article3. Nevadunsky NS, Van Arsdale A, Strickler HD, Moadel A, Kaur G, Levitt J, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014; 124:300–6.
Article4. Salvesen HB, Akslen LA, Iversen T, Iversen OE. Recurrence of endometrial carcinoma and the value of routine follow up. Br J Obstet Gynaecol. 1997; 104:1302–7.
Article5. Leeson S, Stuart N, Sylvestre Y, Hall L, Whitaker R. Gynaecological cancer follow-up: national survey of current practice in the UK. BMJ Open. 2013; 3:e002859.
Article6. Baekelandt MM, Castiglione M. ESMO Guidelines Working Group. Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009; 20:Suppl 4. 29–31.
Article7. Tjalma WA, van Dam PA, Makar AP, Cruickshank DJ. The clinical value and the cost-effectiveness of follow-up in endometrial cancer patients. Int J Gynecol Cancer. 2004; 14:931–7.
Article8. Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br J Cancer. 2012; 107:1195–202.
Article9. Richards M, Corner J, Maher J. The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors. Br J Cancer. 2011; 105:Suppl 1. S1–4.
Article10. MacmillianCancer Support & NHS Improvement. Living with and beyond cancer: taking action to improve outcomes. London: National Cancer Survivorship Initiative;2013.11. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000; 182:1506–19.
Article12. Scholten AN, van Putten WL, Beerman H, Smit VT, Koper PC, Lybeert ML, et al. Postoperative radiotherapy for stage 1 endometrial carcinoma: long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys. 2005; 63:834–8.
Article13. Topfedaisi Ozkan N, Meydanlı MM, Sarı ME, Demirkiran F, Kahramanoglu I, Bese T, et al. Factors associated with survival after relapse in patients with low-risk endometrial cancer treated with surgery alone. J Gynecol Oncol. 2017; 28:e65.
Article14. Jeppesen MM, Mogensen O, Hansen DG, Iachina M, Korsholm M, Jensen PT. Detection of recurrence in early stage endometrial cancer-the role of symptoms and routine follow-up. Acta Oncol. 2017; 56:262–9.15. Shumsky AG, Stuart GC, Brasher PM, Nation JG, Robertson DI, Sangkarat S. An evaluation of routine follow-up of patients treated for endometrial carcinoma. Gynecol Oncol. 1994; 55:229–33.
Article16. Agboola OO, Grunfeld E, Coyle D, Perry GA. Costs and benefits of routine follow-up after curative treatment for endometrial cancer. CMAJ. 1997; 157:879–86.17. Gadducci A, Cosio S, Fanucchi A, Cristofani R, Genazzani AR. An intensive follow-up does not change survival of patients with clinical stage I endometrial cancer. Anticancer Res. 2000; 20:1977–84.18. Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T, et al. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006; 101:520–9.
Article19. Sartori E, Pasinetti B, Chiudinelli F, Gadducci A, Landoni F, Maggino T, et al. Surveillance procedures for patients treated for endometrial cancer: a review of the literature. Int J Gynecol Cancer. 2010; 20:985–92.
Article20. Bendifallah S, Ouldamer L, Lavoue V, Canlorbe G, Raimond E, Coutant C, et al. Patterns of recurrence and outcomes in surgically treated women with endometrial cancer according to ESMO-ESGO-ESTRO Consensus Conference risk groups: results from the FRANCOGYN study Group. Gynecol Oncol. 2017; 144:107–12.
Article21. Francis SR, Ager BJ, Do OA, Huang YJ, Soisson AP, Dodson MK, et al. Recurrent early stage endometrial cancer: patterns of recurrence and results of salvage therapy. Gynecol Oncol. 2019; 154:38–44.
Article22. Ignatov T, Eggemann H, Costa SD, Ortmann O, Ignatov A. Endometrial cancer subtypes are associated with different patterns of recurrence. J Cancer Res Clin Oncol. 2018; 144:2011–7.
Article23. Jeppesen MM, Jensen PT, Gilså Hansen D, Iachina M, Mogensen O. The nature of early-stage endometrial cancer recurrence-A national cohort study. Eur J Cancer. 2016; 69:51–60.
Article24. Beaver K, Williamson S, Sutton C, Hollingworth W, Gardner A, Allton B, et al. Comparing hospital and telephone follow-up for patients treated for stage-I endometrial cancer (ENDCAT trial): a randomised, multicentre, non-inferiority trial. BJOG. 2017; 124:150–60.
Article25. Williamson S, Beaver K, Gardner A, Martin-Hirsch P. Telephone follow-up after treatment for endometrial cancer: a qualitative study of patients’ and clinical nurse specialists’ experiences in the ENDCAT trial. Eur J Oncol Nurs. 2018; 34:61–7.
Article26. Dixon P, Beaver K, Williamson S, Sutton C, Martin-Hirsch P, Hollingworth W. Cost-consequence analysis alongside a randomised controlled trial of hospital versus telephone follow-up after treatment for endometrial cancer. Appl Health Econ Health Policy. 2018; 16:415–27.
Article27. Jeppesen MM, Jensen PT, Hansen DG, Christensen RD, Mogensen O. Patient-initiated follow up affects fear of recurrence and healthcare use: a randomised trial in early-stage endometrial cancer. BJOG. 2018; 125:1705–14.
Article28. Independent Cancer Task Force. Achieving worldclass cancer outcomes: a strategy for England 2015–2020. [Internet]. London (UK): National Health Service;c2020. [cited 2021 Jan 15]. Available from: https://www.eng-land.nhs.uk/wp-content/uploads/2016/10/cancer-one-year-on.pdf .29. Watson EK, Rose PW, Neal RD, Hulbert-Williams N, Donnelly P, Hubbard G, et al. Personalised cancer follow-up: risk stratification, needs assessment or both? Br J Cancer. 2012; 106:1–5.
Article30. Kimman ML, Bloebaum MM, Dirksen CD, Houben RM, Lambin P, Boersma LJ. Patient satisfaction with nurse-led telephone follow-up after curative treatment for breast cancer. BMC Cancer. 2010; 10:174.
Article31. Williamson S, Chalmers K, Beaver K. Patient experiences of nurse-led telephone follow-up following treatment for colorectal cancer. Eur J Oncol Nurs. 2015; 19:237–43.
Article32. Kumarakulasingam P, McDermott H, Patel N, Boutler L, Tincello DG, Peel D, et al. Acceptability and utilisation of patient-initiated follow-up for endometrial cancer amongst women from diverse ethnic and social backgrounds: a mixed methods study. Eur J Cancer Care (Engl). 2019; 28:e12997.
Article33. Newton C, Nordin A, Rolland P, Ind T, Larsen-Disney P, Martin-Hirsch P, et al. British Gynaecological Cancer Society recommendations and guidance on patient-initiated follow-up (PIFU). Int J Gynecol Cancer. 2020; 30:695–700.
Article34. Coleman L, Newton C. Patient initiated follow up after gynaecological malignancy: national survey of current UK practice. Eur J Obstet Gynecol Reprod Biol. 2020; 248:193–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of adjuvant treatment on survival in patients with 2023 FIGO stage IIC endometrial cancer: a retrospective analysis from two tertiary centers in Korea and Taiwan
- Medically unfit women with early-stage endometrial cancer treated with the levonorgestrel intrauterine system
- 3 Cases of Synchronous Primary Carcinomas
- Clinical data of patients with endometrioid endometrial adenocarcinoma at a single institution
- A case of simultaneous presentation of uterine endometrial adenocarcinoma with right ovarian endometrioid carcinoma and left ovarian serous adenocarcinoma