Healthc Inform Res.
2021 Oct;27(4):279-286. 10.4258/hir.2021.27.4.279.
Estimating the Optimal Dexketoprofen Pharmaceutical Formulation with Machine Learning Methods and Statistical Approaches
- Affiliations
-
- 1Department of Big Data Analytics and Management, Institute of Science and Technology, Bahcesehir University, Istanbul, Turkey
- 2Department of Pharmaceutical Technology, Faculty of Pharmacy, Istanbul University, Istanbul, Turkey
- 3Department of Software Engineering, Faculty of Engineering and Natural Sciences, Bahcesehir University, Istanbul, Turkey
- KMID: 2522209
- DOI: http://doi.org/10.4258/hir.2021.27.4.279
Abstract
Objectives
Orally disintegrating tablets (ODTs) can be utilized without any drinking water; this feature makes ODTs easy to use and suitable for specific groups of patients. Oral administration of drugs is the most commonly used route, and tablets constitute the most preferable pharmaceutical dosage form. However, the preparation of ODTs is costly and requires long trials, which creates obstacles for dosage trials. The aim of this study was to identify the most appropriate formulation using machine learning (ML) models of ODT dexketoprofen formulations, with the goal of providing a cost-effective and timereducing solution.
Methods
This research utilized nonlinear regression models, including the k-nearest neighborhood (k-NN), support vector regression (SVR), classification and regression tree (CART), bootstrap aggregating (bagging), random forest (RF), gradient boosting machine (GBM), and extreme gradient boosting (XGBoost) methods, as well as the t-test, to predict the quantity of various components in the dexketoprofen formulation within fixed criteria.
Results
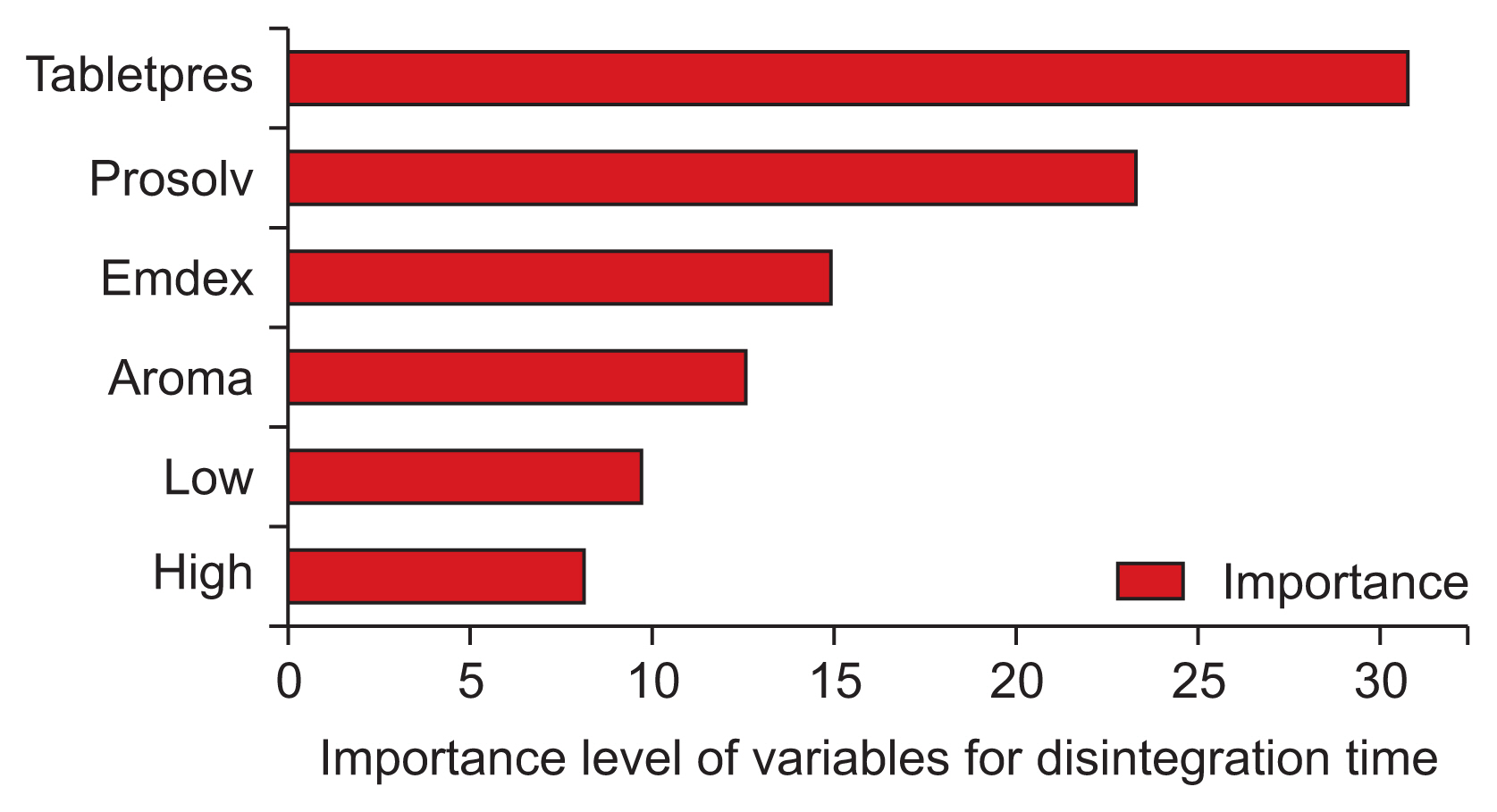
All the models were developed with Python libraries. The performance of the ML models was evaluated with R2 values and the root mean square error. Hardness values of 0.99 and 2.88, friability values of 0.92 and 0.02, and disintegration time values of 0.97 and 10.09 using the GBM algorithm gave the best results.
Conclusions
In this study, we developed a computational approach to estimate the optimal pharmaceutical formulation of dexketoprofen. The results were evaluated by an expert, and it was found that they complied with Food and Drug Administration criteria.
Keyword
Figure
Reference
-
References
1. Ezcurdia M, Cortejoso FJ, Lanzon R, Ugalde FJ, Herruzo A, Artigas R, et al. Comparison of the efficacy and tolerability of dexketoprofen and ketoprofen in the treatment of primary dysmenorrhea. J Clin Pharmacol. 1998; 38(S1):65S–73S.
Article2. Kara I, Tuncer S, Erol A, Reisli R. [The effects of preemptive dexketoprofen use on postoperative pain relief and tramadol consumption]. Agri. 2011; 23(1):18–21.3. Mesut B, Aksu N, Ozsoy Y. Design of sustained release tablet formulations of alfuzosin HCl by means of neurofuzzy logic. Lat Am J Pharmacy. 2013; 32(9):1288–97.4. Dere S, Ayvaz S. Prediction of drug-drug interactions by using profile fingerprint vectors and protein similarities. Healthc Inform Res. 2020; 26(1):42–9.
Article5. Ahamad MM, Aktar S, Rashed-Al-Mahfuz M, Uddin S, Lio P, Xu H, et al. A machine learning model to identify early stage symptoms of SARS-Cov-2 infected patients. Expert Syst Appl. 2020; 160:113661.
Article6. Amiri MM, Tapak L, Faradmal J, Hosseini J, Roshanaei G. Prediction of serum creatinine in hemodialysis patients using a kernel approach for longitudinal data. Healthc Inform Res. 2020; 26(2):112–8.
Article7. Vetter TR. Fundamentals of research data and variables: the devil is in the details. Anesth Analg. 2017; 125(4):1375–80.8. Kim TK. T test as a parametric statistic. Korean J Anesthesiol. 2015; 68(6):540–6.
Article9. McKnight PE, Najab J. Mann-Whitney U test. In : Weiner IB, Edward Craighead W, editors. The Corsini encyclopedia of psychology. Hoboken (NJ): John Wiley & Sons;2010.10. Adebowale A, Idowu SA, Amarachi A. Comparative study of selected data mining algorithms used for intrusion detection. Int J Soft Comput Eng. 2013; 3(3):237–41.11. Gunn SR. Support vector machines for classification and regression. Southampton, UK: University of Southampton;1998.12. Wu X, Kumar V, Quinlan JR, Ghosh J, Yang Q, Motoda H, et al. Top 10 algorithms in data mining. Knowl Inf Syst. 2008; 14(1):1–37.
Article13. Breiman L. Bagging predictors. Mach Learn. 1996; 24(2):123–40.
Article14. Breiman L. Random forests. Mach Learn. 2001; 45(1):5–32.15. Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat. 2001; 29(5):1189–232.
Article16. Chen T, Guestrin C. XgBoost: a scalable tree boosting system. In : Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; 2016 Aug 13–17; San Francisco, CA. p. 785–94.17. Probst P, Boulesteix AL, Bischl B. Tunability: importance of hyperparameters of machine learning algorithms. J Mach Learn Res. 2019; 20(1):1934–65.18. Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012; 10(2):486–9.
Article19. O’Neill ME, Mathews KL. Levene tests of homogeneity of variance for general block and treatment designs. Biometrics. 2002; 58(1):216–24.20. US Food and Drug Administration. Q6A specifications: test procedures and acceptance criteria for new drug substances and new drug products: chemical substances [Internet]. Silver Spring (MD): Food and Drug Administration;2000. [cited at 2021 Oct 10]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q6a-specifications-test-procedures-and-acceptance-criteria-new-drug-substances-and-new-drug-products .21. European Medicines Agency. ICH topic Q6a specifications: Test procedures and acceptance criteria for new drug substances and new drug products: Chemical substances [Internet]. London, UK: European Medicines Agency;2000. [cited at 2021 Oct 10]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-6-test-procedures-acceptance-criteria-new-drug-substances-new-drug-products-chemical_en.pdf .22. Mesut B, Baskor A, Pirincci Tok Y, Alkan B, Erginer Y. Statistical investigation of the effect of excipients particle size on orally disintegrating tablets: mannitol grades. Informatica. 2020; 31(7):69–91.23. Center for Drug Evaluation and Research. Scale-up and postapproval changes: chemistry, manufacturing, and controls: in vitro dissolution testing, and in vivo bioequivalence documentation [Internet]. Silver Spring (MD): Food and Drug Administration;1995. [cited at 2021 Oct 10]. Available from: https://www.fda.gov/media/70949/download .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Machine Learning vs. Statistical Model for Prediction Modelling: Application in Medical Imaging Research
- Review of statistical methods for survival analysis using genomic data
- A Review of Machine Learning Approaches for Brain Positron Emission Tomography Data Analysis
- Prediction of Diabetic Neuropathy Using Machine Learning Techniques
- Machine Learning and Deep Learning for the Pharmacogenomics of Antidepressant Treatments