Clin Endosc.
2021 Jul;54(4):613-617. 10.5946/ce.2020.241.
Micro-Biopsy Forceps in the Assessment of Peritoneal Carcinomatosis: A Possible New Indication?
- Affiliations
-
- 1Gastroenterology and Digestive Endoscopy Unit, Forlì - Cesena Hospitals, AUSL Romagna, Forlì-Cesena, Italy
- 2Gastroenterology and Interventional Endoscopy Unit, Ospedale Maggiore “C.A. Pizzardi”, AUSL Bologna, Bologna, Italy
- 3Servizio di Endoscopia Digestiva, Fondazione Istituto G. Giglio di Cefalù, Cefalù (PA), Italy
- 4Section of Nutrition, Istituto Euromediterraneo di Scienze e Tecnologia (IEMEST), Palermo, Italy
- 5Pathology Unit, Ospedale Maggiore “C.A. Pizzardi”, AUSL Bologna, Bologna, Italy
- 6Pathology Unit, Morgagni-Pierantoni Hospital, Forlì, AUSL Romagna, Forlì, Italy
- 7Digestive Endoscopy Unit, Humanitas Clinical and Research Center – IRCCS, Rozzano (MI), Italy
- KMID: 2518867
- DOI: http://doi.org/10.5946/ce.2020.241
Abstract
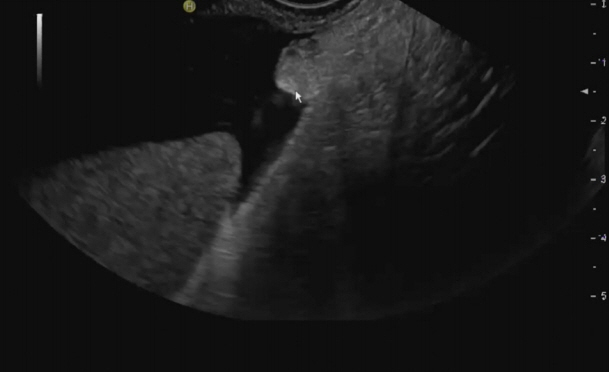
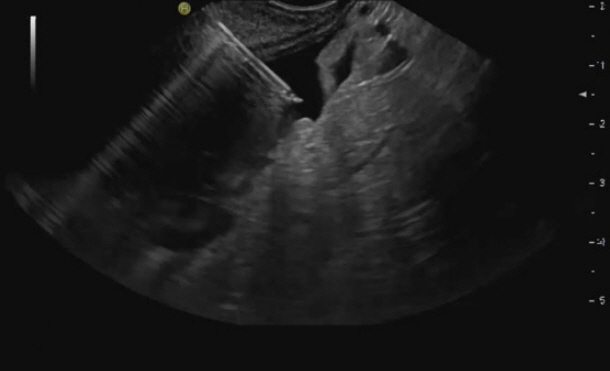
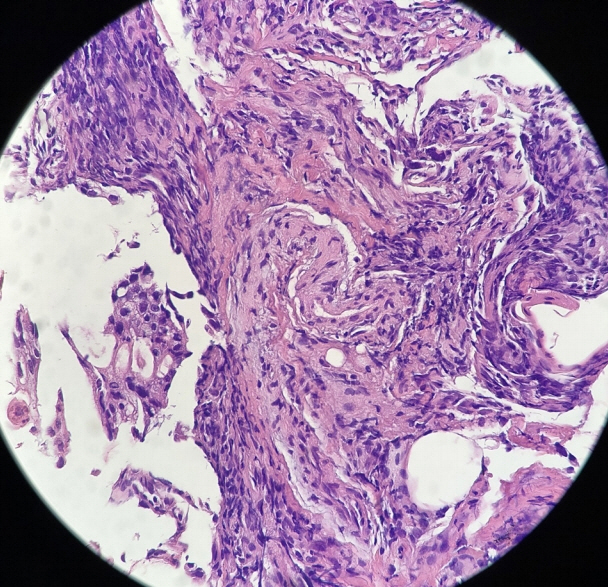
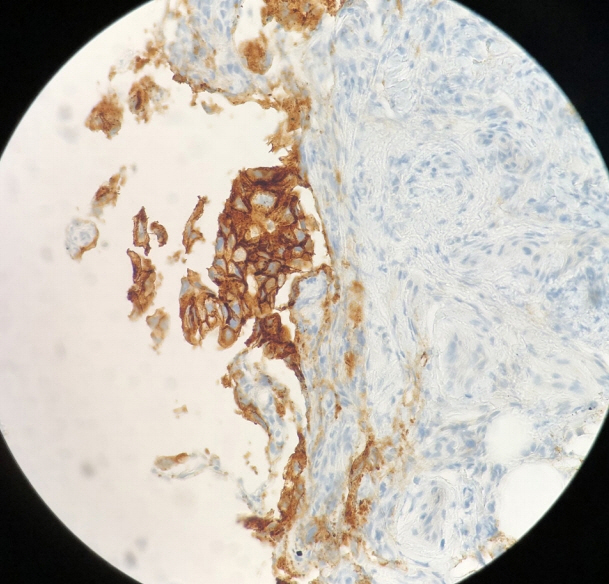
- Peritoneal carcinomatosis (PC) is defined as a metastatic involvement of the peritoneum by several other primary sites and it is characterized by a marked worsening of prognosis, with limited treatment opportunities. Subsequently, PC should be ruled out before any invasive treatment is administered. A new through-the-needle micro-biopsy forceps (MF) was recently introduced that permits micro-histology cores. In this case series, we evaluated the feasibility of MF in the assessment of PC to complete patient diagnostic work-ups. Five consecutive patients referred for endoscopic ultrasound staging were sampled using MF. Sampling was feasible in all patients with a technical success of 100%. No adverse events were reported in any cases. This technique was feasible and safe with a technical success rate of 100%. It permitted sampling of peritoneal irregularity, obtained high-quality tissue fragments in all cases, and enabled an additional assessment, i.e., immunohistochemical staining.
Figure
Reference
-
1. Terzi C, Arslan NC, Canda AE. Peritoneal carcinomatosis of gastrointestinal tumors: where are we now? World J Gastroenterol. 2014; 20:14371–14380.
Article2. Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JWW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011; 128:2717–2725.
Article3. Patel CM, Sahdev A, Reznek RH. CT, MRI and PET imaging in peritoneal malignancy. Cancer Imaging. 2011; 11:123–139.
Article4. Lobo C, Costa J, Petronilho S, Monteiro P, Leça L, Schmitt F. Cytohistological correlation in serous effusions using the newly proposed international system for reporting serous fluid cytopathology: experience of an oncological center. Diagn Cytopathol. 2021; 49:596–605.5. Rana SS, Bhasin DK, Srinivasan R, Singh K. Endoscopic ultrasound-guided fine needle aspiration of peritoneal nodules in patients with ascites of unknown cause. Endoscopy. 2011; 43:1010–1013.
Article6. Levy MJ, Abu Dayyeh BK, Fujii LL, et al. Detection of peritoneal carcinomatosis by EUS fine-needle aspiration: impact on staging and resectability (with videos). Gastrointest Endosc. 2015; 81:1215–1224.
Article7. Kovacevic B, Karstensen JG, Havre RF, et al. Initial experience with EUS-guided microbiopsy forceps in diagnosing pancreatic cystic lesions: a multicenter feasibility study (with video). Endosc Ultrasound. 2018; 7:383–388.
Article8. Barresi L, Crinò SF, Fabbri C, et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A multicenter study. Dig Endosc. 2018; 30:760–770.
Article9. Zhang ML, Arpin RN, Brugge WR, Forcione DG, Basar O, Pitman MB. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018; 126:414–420.
Article10. Crinò SF, Bernardoni L, Brozzi L, et al. Association between macroscopically visible tissue samples and diagnostic accuracy of EUS-guided through-the-needle microforceps biopsy sampling of pancreatic cystic lesions. Gastrointest Endosc. 2019; 90:933–943.
Article11. Sharma V, Rana SS, Ahmed SU, Guleria S, Sharma R, Gupta R. Endoscopic ultrasound-guided fine-needle aspiration from ascites and peritoneal nodules: a scoping review. Endosc Ultrasound. 2017; 6:382–388.
Article12. Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001; 54:336–339.
Article13. Facciorusso A, Del Prete V, Antonino M, Buccino VR, Wani S. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: a meta-analysis. Gastrointest Endosc. 2020; 92:1–8. e3.
Article14. Tacelli M, Celsa C, Magro B, et al. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: systematic review with meta-analysis. Dig Endosc. 2020; 32:1018–1030.
Article15. Crinò SF, Bernardoni L, Brozzi L, et al. Association between macroscopically visible tissue samples and diagnostic accuracy of EUS-guided through-the-needle microforceps biopsy sampling of pancreatic cystic lesions. Gastrointest Endosc. 2019; 90:933–943.
Article16. Larghi A, Manfrin E, Fabbri C, et al. Interobserver agreement among expert pathologists on through-the-needle microforceps biopsy samples for evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2019; 90:784–792. e4.
Article17. Li C, Shuai Y, Zhou X. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of intra-abdominal lymphadenopathy: a systematic review and meta-analysis. Scand J Gastroenterol. 2020; 55:114–122.
Article18. Saito K, Nakai Y, Ushiku T, et al. Gastrointestinal: successful diagnosis of primary peritoneal serous carcinoma by endoscopic ultrasound-guided through-the-needle forceps biopsy. J Gastroenterol Hepatol. 2019; 34:1271.
Article19. Kocaman O, Danalioğlu A, İnce AT, Tozlu M, Şentürk H. Diagnosis of tuberculous peritonitis using endoscopic ultrasound-guided fine-needle aspiration biopsy of the peritoneum. Turk J Gastroenterol. 2013; 24:65–69.
Article20. Peter S, Eltoum I, Eloubeidi MA. EUS-guided FNA of peritoneal carcinomatosis in patients with unknown primary malignancy. Gastrointest Endosc. 2009; 70:1266–1270.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Abdominal Sarcoidosis Mimicking Peritoneal Carcinomatosis
- Gastric Cancer with Peritoneal Tuberculosis: Challenges in Diagnosis and Treatment
- Peritoneal carcinomatosis mimicking a peritoneal tuberculosis
- Treatment of Peritoneal Carcinomatosis from Colorectal Cancer
- Prognostic Factors in Advanced Gastric Cancer with Peritoneal Carcinomatosis