J Pathol Transl Med.
2021 Jul;55(4):279-288. 10.4132/jptm.2021.05.10.
Correlation of TTF-1 immunoexpression and EGFR mutation spectrum in non–small cell lung carcinoma
- Affiliations
-
- 1Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
- 2Department of Medical Oncology, Dr B.R. Ambedkar Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Pulmonary Medicine and Sleep Disorders, All India Institute of Medical Sciences, New Delhi, India
- KMID: 2518428
- DOI: http://doi.org/10.4132/jptm.2021.05.10
Abstract
- Background
Thyroid transcription factor (TTF-1) is a diagnostic marker expressed in 75%–85% of primary lung adenocarcinomas (ACs). Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) gene is the most common targetable driver alteration in lung AC. Previous studies have shown a positive correlation between TTF-1 and EGFR mutation status. We aimed to determine the predictive value of TTF-1 immunoexpression for underlying EGFR mutation status in a large Indian cohort.
Methods
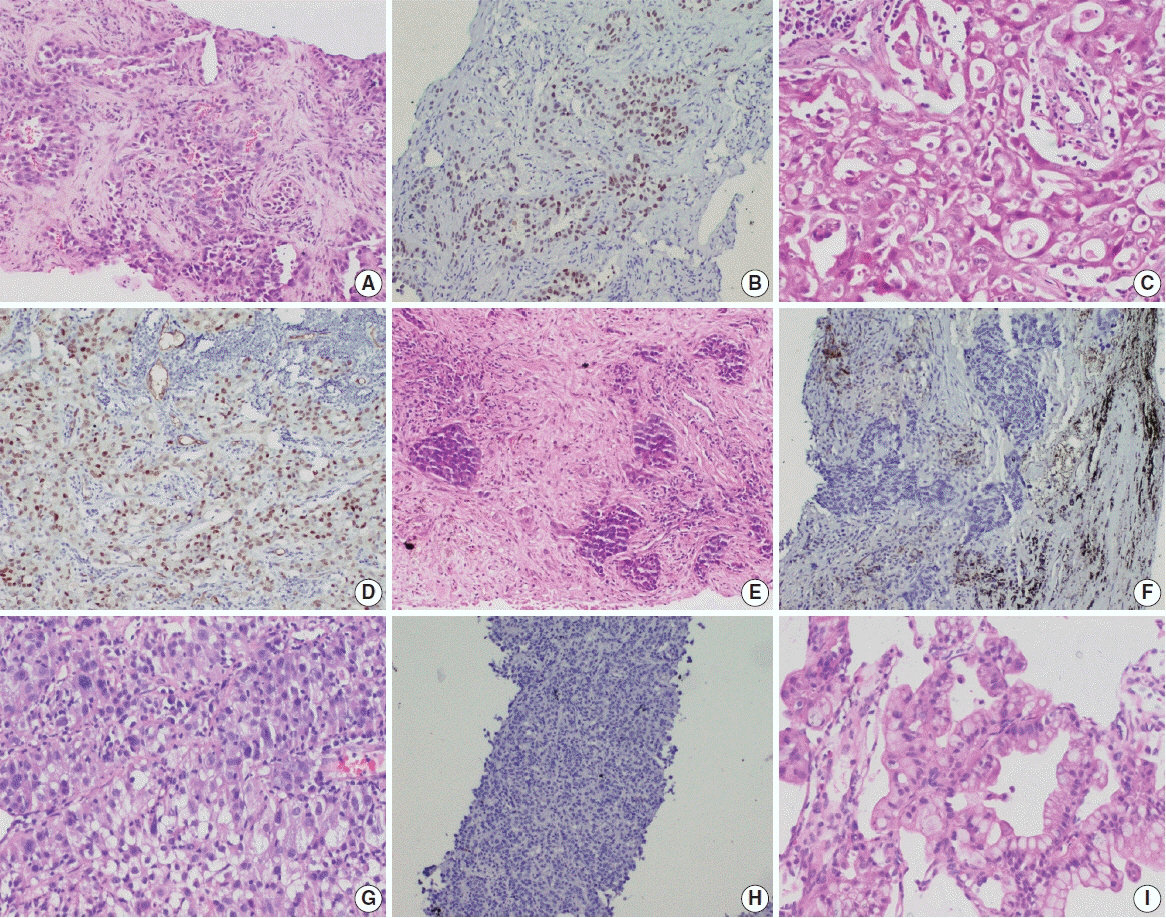
This retrospective designed study was conducted with medical record data from 2011 to 2020. All cases of primary lung AC and non–small cell lung carcinoma not otherwise specified (NSCLC, NOS) with known TTF-1 expression diagnosed by immunohistochemistry using 8G7G3/1 antibodies and EGFR mutation status diagnosed by quantitative polymerase chain reaction were retrieved, reviewed, and the
results
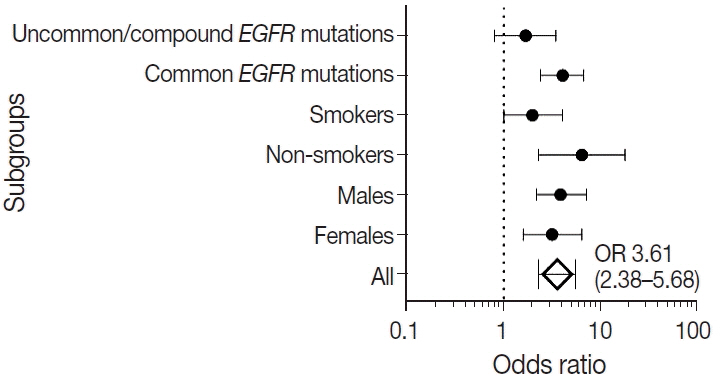
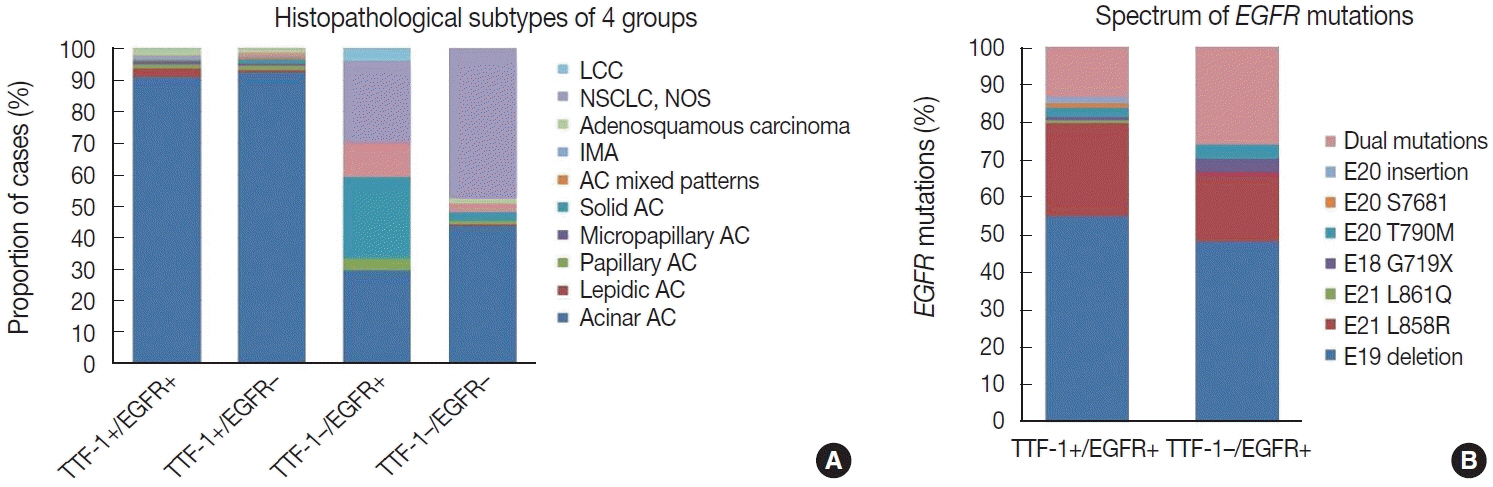
were analyzed. Results: Among 909 patient samples diagnosed as lung AC and NSCLC, NOS, TTF-1 was positive in 76.8% cases (698/909) and EGFR mutations were detected in 29.6% (269/909). A strong positive correlation was present between TTF-1 positivity and EGFR mutation status (odds ratio, 3.61; p < .001), with TTF-1 positivity showing high sensitivity (90%) and negative predictive value (87%) for EGFR mutation. TTF-1 immunoexpression did not show significant correlation with uncommon/dual EGFR mutations (odds ratio, 1.69; p = .098). EGFR–tyrosine kinase inhibitor therapy was significantly superior to chemotherapy among EGFR mutant cases irrespective of TTF-1 status; however, no significant differences among survival outcomes were observed.
Conclusions
Our study confirms a strong positive correlation between TTF-1 expression and common EGFR mutations (exon 19 deletion and exon 21 L858R) in advanced lung AC with significantly high negative predictive value of TTF-1 for EGFR mutations.
Keyword
Figure
Reference
-
References
1. Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol. 2002; 26:767–73.
Article2. Lau SK, Luthringer DJ, Eisen RN. Thyroid transcription factor-1: a review. Appl Immunohistochem Mol Morphol. 2002; 10:97–102.
Article3. Walia R, Jain D, Madan K, et al. p40 & thyroid transcription factor- 1 immunohistochemistry: a useful panel to characterize nonsmall cell lung carcinoma-not otherwise specified (NSCLC-NOS) category. Indian J Med Res. 2017; 146:42–8.4. Yatabe Y, Dacic S, Borczuk AC, et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol. 2019; 14:377–407.
Article5. Malik PS, Sharma MC, Mohanti BK, et al. Clinico-pathological profile of lung cancer at AIIMS: a changing paradigm in India. Asian Pac J Cancer Prev. 2013; 14:489–94.
Article6. Noronha V, Pinninti R, Patil VM, Joshi A, Prabhash K. Lung cancer in the Indian subcontinent. South Asian J Cancer. 2016; 5:95–103.
Article7. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the study of lung cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018; 13:323–58.8. Jain D, Iqbal S, Walia R, et al. Evaluation of epidermal growth factor receptor mutations based on mutation specific immunohistochemistry in non-small cell lung cancer: a preliminary study. Indian J Med Res. 2016; 143:308–14.
Article9. Inamura K. Update on immunohistochemistry for the diagnosis of lung cancer. Cancers (Basel). 2018; 10:72.
Article10. Suda K, Mitsudomi T. Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch Toxicol. 2015; 89:1227–40.
Article11. Berghmans T, Paesmans M, Mascaux C, et al. Thyroid transcription factor 1: a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol. 2006; 17:1673–6.12. Chung KP, Huang YT, Chang YL, et al. Clinical significance of thyroid transcription factor-1 in advanced lung adenocarcinoma under epidermal growth factor receptor tyrosine kinase inhibitor treatment. Chest. 2012; 141:420–8.
Article13. Nakra T, Mehta A, Bal A, et al. Epidermal growth factor receptor mutation status in pulmonary adenocarcinoma: multi-institutional data discussion at national conference of “Lung Cancer Management in Indian context”. Curr Probl Cancer. 2020; 44:100561.
Article14. Singh V, Guleria P, Malik PS, et al. Epidermal growth factor receptor (EGFR), KRAS, and BRAF mutations in lung adenocarcinomas: a study from India. Curr Probl Cancer. 2019; 43:391–401.15. Singh V, Nambirajan A, Malik PS, et al. Spectrum of uncommon and compound epidermal growth factor receptor mutations in nonsmall- cell lung carcinomas with treatment response and outcome analysis: a study from India. Lung Cancer. 2020; 149:53–60.16. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th. Lyon: IARC Press;2015.17. Sheffield BS, Bosdet IE, Ali RH, et al. Relationship of thyroid transcription factor 1 to EGFR status in non-small-cell lung cancer. Curr Oncol. 2014; 21:305–8.18. Carneiro JG, Couto PG, Bastos-Rodrigues L, et al. Spectrum of somatic EGFR, KRAS, BRAF, PTEN mutations and TTF-1 expression in Brazilian lung cancer patients. Genet Res (Camb). 2014; 96:e002.
Article19. Udupa KS, Rajendranath R, Sagar TG, Sundersingh S, Joseph T. Dual surrogate markers for rapid prediction of epidermal growth factor receptor mutation status in advanced adenocarcinoma of the lung: a novel approach in resource-limited setting. Indian J Cancer. 2015; 52:266–8.
Article20. Sun PL, Seol H, Lee HJ, et al. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF- 1 expressions, and clinical features. J Thorac Oncol. 2012; 7:323–30.21. Zhao Q, Xu S, Liu J, et al. Thyroid transcription factor-1 expression is significantly associated with mutations in exon 21 of the epidermal growth factor receptor gene in Chinese patients with lung adenocarcinoma. Onco Targets Ther. 2015; 8:2469–78.22. Gahr S, Stoehr R, Geissinger E, et al. EGFR mutational status in a large series of Caucasian European NSCLC patients: data from daily practice. Br J Cancer. 2013; 109:1821–8.23. Kim HS, Kim JH, Han B, Choi DR. Correlation of thyroid transcription factor-1 expression with EGFR mutations in non-smallcell lung cancer: a meta-analysis. Medicina (Kaunas). 2019; 55:41.24. Travis WD, Noguchi M, Yatabe Y, et al. Adenocarcinoma. In : Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: IARC Press;2015. p. 46–51.25. Shanzhi W, Yiping H, Ling H, Jianming Z, Qiang L. The relationship between TTF-1 expression and EGFR mutations in lung adenocarcinomas. PLoS One. 2014; 9:e95479.26. Dong YJ, Cai YR, Zhou LJ, et al. Association between the histological subtype of lung adenocarcinoma, EGFR/KRAS mutation status and the ALK rearrangement according to the novel IASLC/ATS/ERS classification. Oncol Lett. 2016; 11:2552–8.27. Wei WE, Mao NQ, Ning SF, et al. An analysis of EGFR mutations among 1506 cases of non-small cell lung xancer patients in Guangxi, China. PLoS One. 2016; 11:e0168795.28. Park JY, Jang SH, Kim HI, et al. Thyroid transcription factor-1 as a prognostic indicator for stage IV lung adenocarcinoma with and without EGFR-sensitizing mutations. BMC Cancer. 2019; 19:574.
Article29. Vallee A, Sagan C, Le Loupp AG, Bach K, Dejoie T, Denis MG. Detection of EGFR gene mutations in non-small cell lung cancer: lessons from a single-institution routine analysis of 1,403 tumor samples. Int J Oncol. 2013; 43:1045–51.30. Somaiah N, Fidler MJ, Garrett-Mayer E, et al. Epidermal growth factor receptor (EGFR) mutations are exceptionally rare in thyroid transcription factor (TTF-1)-negative adenocarcinomas of the lung. Oncoscience. 2014; 1:522–8.31. Vincenten J, Smit EF, Vos W, et al. Negative NKX2-1 (TTF-1) as temporary surrogate marker for treatment selection during EGFRmutation analysis in patients with non-small-cell lung cancer. J Thorac Oncol. 2012; 7:1522–7.32. Jie L, Li XY, Zhao YQ, et al. Genotype-phenotype correlation in Chinese patients with pulmonary mixed type adenocarcinoma: relationship between histologic subtypes, TITF-1/SP-A expressions and EGFR mutations. Pathol Res Pract. 2014; 210:176–81.33. Lange K, Brunner E. Analysis of predictive values based on individual risk factors in multi-modality trials. Diagnostics (Basel). 2013; 3:192–209.
Article34. Gaber R, Goldmann T. Mini review: immunohistochemistry using EGFR-mutant specific antibodies in non-small cell lung carcinoma: accuracy and reliability. J Cancer Treatment Diagn. 2018; 2:17–23.35. Jiang G, Fan C, Zhang X, et al. Ascertaining an appropriate diagnostic algorithm using EGFR mutation-specific antibodies to detect EGFR status in non-small-cell lung cancer. PLoS One. 2013; 8:e59183.36. Gazdar AF. Activating and resistance mutations of EGFR in nonsmall- cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009; 28 Suppl 1:S24–31.37. Nan X, Xie C, Yu X, Liu J. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget. 2017; 8:75712–26.38. Nakahara Y, Hosomi Y, Saito M, et al. Predictive significance of thyroid transcription factor-1 expression in patients with non-squamous non-small cell lung cancer with wild-type epidermal growth factor receptor treated with erlotinib. Mol Clin Oncol. 2016; 5:14–8.
Article39. Sun JM, Han J, Ahn JS, Park K, Ahn MJ. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed- based chemotherapy. J Thorac Oncol. 2011; 6:1392–9.40. Tang X, Kadara H, Behrens C, et al. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res. 2011; 17:2434–43.
Article41. Yamaguchi T, Yanagisawa K, Sugiyama R, et al. NKX2-1/TITF1/TTF-1-induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012; 21:348–61.42. Kret A, Clark C, Tennant S, Nicolson M, Kerr KM. Mutation analysis and association with TTF1 expression in lung non-squamous NSCLC. Lung Cancer. 2015; 87(Suppl 1):S16.43. Gronberg BH, Lund-Iversen M, Strom EH, Brustugun OT, Scott H. Associations between TS, TTF-1, FR-alpha, FPGS, and overall survival in patients with advanced non-small-cell lung cancer receiving pemetrexed plus carboplatin or gemcitabine plus carboplatin as first-line chemotherapy. J Thorac Oncol. 2013; 8:1255–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidermal Growth Factor Receptor Expression of Non-small Cell Carcinoma and Its Relationship with Genomic Mutation
- Gefitinib Treatment for Pulmonary Sarcomatoid Carcinoma Driven by an EGFR Mutation: Two Cases
- A Case of Favorable Responses after Gefitinib in a Patient with EGFR Mutated Adenosquamous Lung Carcinoma
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- Thyroid Transcription Factor-1 (TTF-1) Expression in Human Lung Carcinomas: Its Prognostic Implication and Relationship with Expressions of p53 and Ki-67 Proteins