J Stroke.
2021 May;23(2):234-243. 10.5853/jos.2020.05064.
Automated Prediction of Ischemic Brain Tissue Fate from Multiphase Computed Tomographic Angiography in Patients with Acute Ischemic Stroke Using Machine Learning
- Affiliations
-
- 1Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
- 2Department of Radiology, University of Calgary, Calgary, AB, Canada
- 3Division of Neuroradiology, Clinic of Radiology and Nuclear Medicine, University Hospital Basel, University of Basel, Basel, Switzerland
- 4Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
- KMID: 2516412
- DOI: http://doi.org/10.5853/jos.2020.05064
Abstract
- Background and Purpose
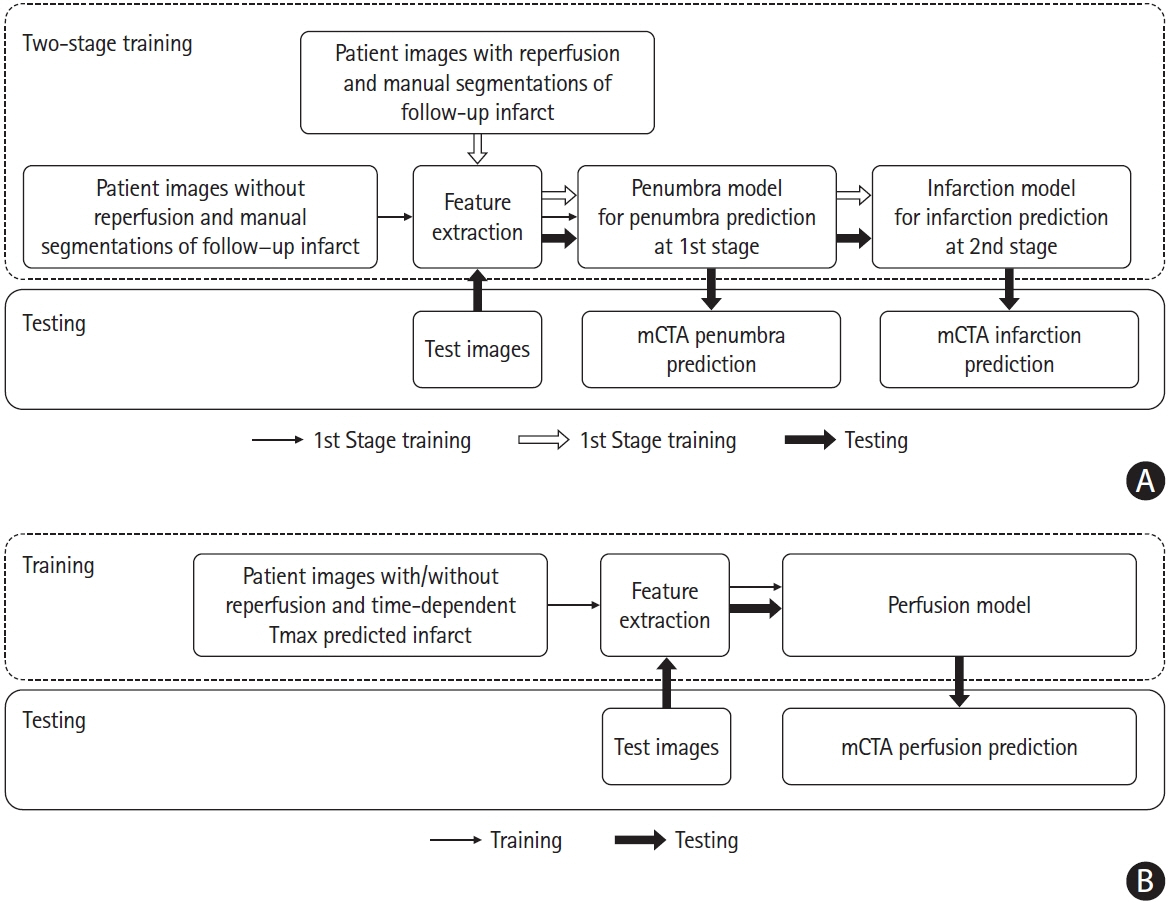
Multiphase computed tomographic angiography (mCTA) provides time variant images of pial vasculature supplying brain in patients with acute ischemic stroke (AIS). To develop a machine learning (ML) technique to predict tissue perfusion and infarction from mCTA source images.
Methods
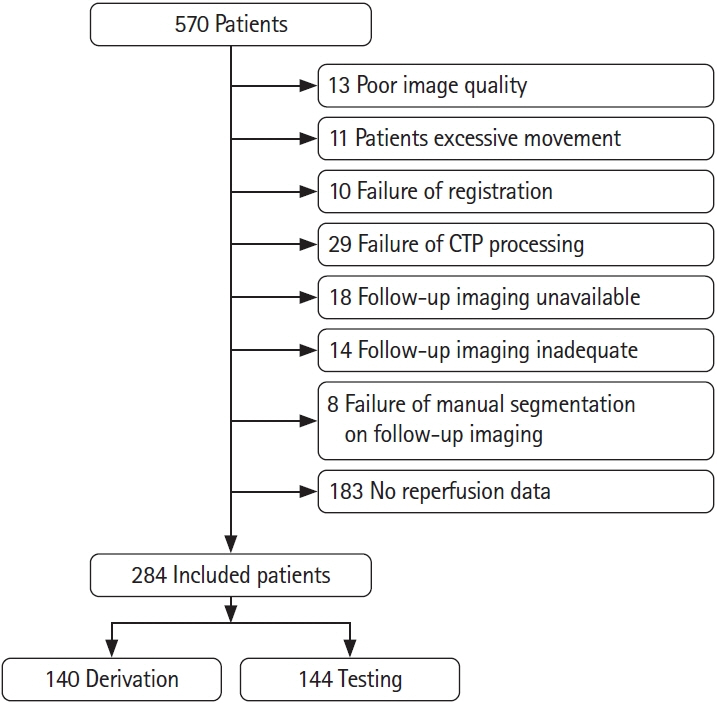
284 patients with AIS were included from the Precise and Rapid assessment of collaterals using multi-phase CTA in the triage of patients with acute ischemic stroke for Intra-artery Therapy (Prove-IT) study. All patients had non-contrast computed tomography, mCTA, and computed tomographic perfusion (CTP) at baseline and follow-up magnetic resonance imaging/non-contrast-enhanced computed tomography. Of the 284 patient images, 140 patient images were randomly selected to train and validate three ML models to predict a pre-defined Tmax thresholded perfusion abnormality, core and penumbra on CTP. The remaining 144 patient images were used to test the ML models. The predicted perfusion, core and penumbra lesions from ML models were compared to CTP perfusion lesion and to follow-up infarct using Bland-Altman plots, concordance correlation coefficient (CCC), intra-class correlation coefficient (ICC), and Dice similarity coefficient.
Results
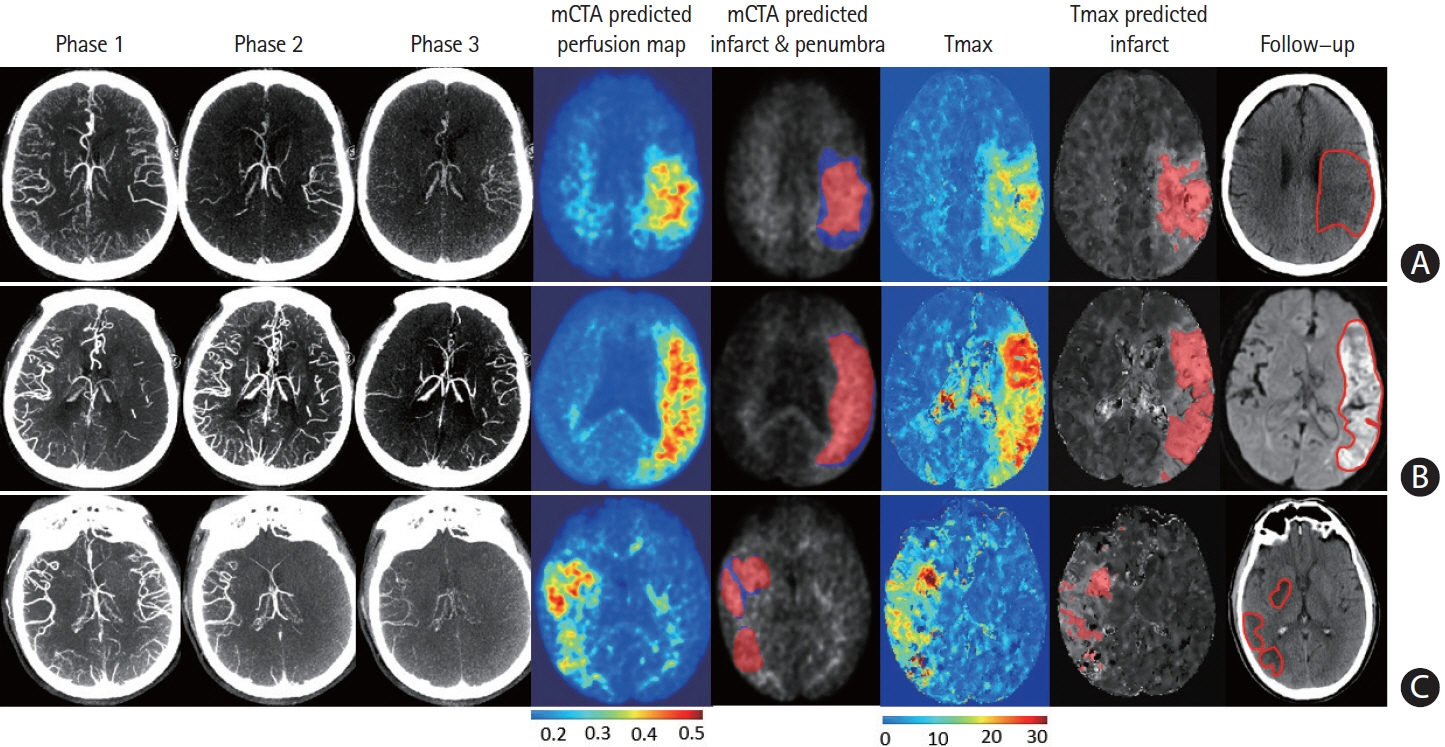
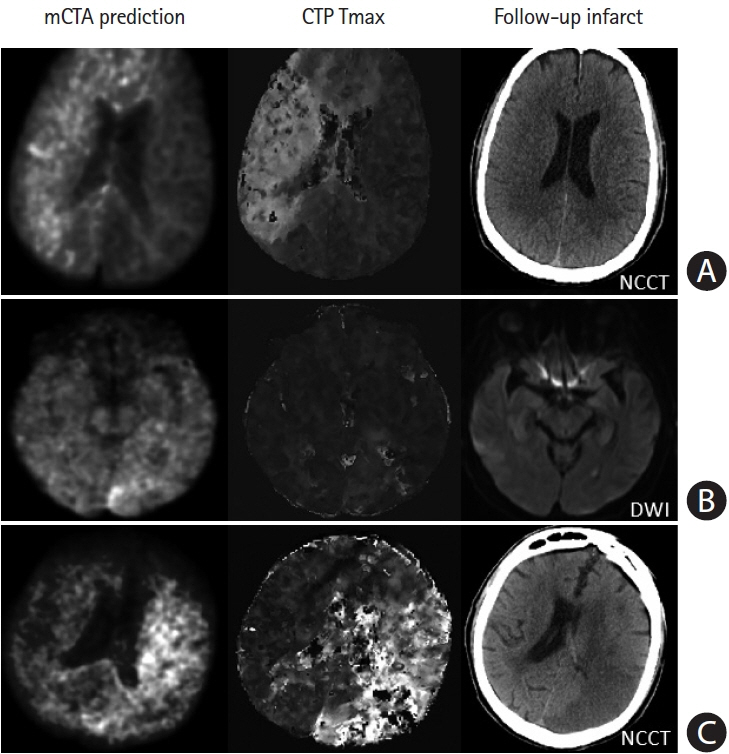
Mean difference between the mCTA predicted perfusion volume and CTP perfusion volume was 4.6 mL (limit of agreement [LoA], –53 to 62.1 mL; P=0.56; CCC 0.63 [95% confidence interval [CI], 0.53 to 0.71; P<0.01], ICC 0.68 [95% CI, 0.58 to 0.78; P<0.001]). Mean difference between the mCTA predicted infarct and follow-up infarct in the 100 patients with acute reperfusion (modified thrombolysis in cerebral infarction [mTICI] 2b/2c/3) was 21.7 mL, while it was 3.4 mL in the 44 patients not achieving reperfusion (mTICI 0/1). Amongst reperfused subjects, CCC was 0.4 (95% CI, 0.15 to 0.55; P<0.01) and ICC was 0.42 (95% CI, 0.18 to 0.50; P<0.01); in non-reperfused subjects CCC was 0.52 (95% CI, 0.20 to 0.60; P<0.001) and ICC was 0.60 (95% CI, 0.37 to 0.76; P<0.001). No difference was observed between the mCTA and CTP predicted infarct volume in the test cohort (P=0.67).
Conclusions
A ML based mCTA model is able to predict brain tissue perfusion abnormality and follow-up infarction, comparable to CTP.
Keyword
Figure
Reference
-
References
1. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega- Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.
Article2. Mokin M, Levy EI, Saver JL, Siddiqui AH, Goyal M, Bonafé A, et al. Predictive value of RAPID assessed perfusion thresholds on final infarct volume in SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke. 2017; 48:932–938.
Article3. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21.4. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion- imaging selection. N Engl J Med. 2015; 372:1009–1018.5. Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011; 42:3435–3440.
Article6. d’Esterre CD, Boesen ME, Ahn SH, Pordeli P, Najm M, Minhas P, et al. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke. 2015; 46:3390–3397.
Article7. Qiu W, Kuang H, Menon BK. Response by Qiu et al to letter regarding article, “Confirmatory study of time-dependent computed tomographic perfusion thresholds for use in acute ischemic stroke”. Stroke. 2020; 51:e8.
Article8. Qiu W, Kuang H, Lee TY, Boers AM, Brown S, Muir K, et al. Confirmatory study of time-dependent computed tomographic perfusion thresholds for use in acute ischemic stroke. Stroke. 2019; 50:3269–3273.
Article9. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.10. Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018; 320:1017–1026.
Article11. Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015; 275:510–520.
Article12. Ospel JM, Volny O, Qiu W, Najm M, Kashani N, Goyal M, et al. Displaying multiphase CT angiography using a time-variant color map: practical considerations and potential applications in patients with acute stroke. AJNR Am J Neuroradiol. 2020; 41:200–205.
Article13. d’Esterre CD, Trivedi A, Pordeli P, Boesen M, Patil S, Hwan Ahn S, et al. Regional comparison of multiphase computed tomographic angiography and computed tomographic perfusion for prediction of tissue fate in ischemic stroke. Stroke. 2017; 48:939–945.
Article14. Yu I, Bang OY, Chung JW, Kim YC, Choi EH, Seo WK, et al. Admission diffusion-weighted imaging lesion volume in patients with large vessel occlusion stroke and Alberta stroke program early CT score of ≥6 points: serial computed tomography- magnetic resonance imaging collateral measurements. Stroke. 2019; 50:3115–3120.15. Lee SJ, Jung WS, Choi MH, Hong JM, Lee JS, Choi JW. Optimal multiphase computed tomographic angiography-based infarct core estimations for acute ischemic stroke. Sci Rep. 2019; 9:15243.
Article16. Najm M, Kuang H, Federico A, Jogiat U, Goyal M, Hill MD, et al. Automated brain extraction from head CT and CTA images using convex optimization with shape propagation. Comput Methods Programs Biomed. 2019; 176:1–8.
Article17. Boers AMM, Jansen IGH, Beenen LFM, Devlin TG, San Roman L, Heo JH, et al. Association of follow-up infarct volume with functional outcome in acute ischemic stroke: a pooled analysis of seven randomized trials. J Neurointerv Surg. 2018; 10:1137–1142.
Article18. Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, et al. Fast free-form deformation using graphics processing units. Comput Methods Programs Biomed. 2010; 98:278–284.
Article19. Bivard A, Kleinig T, Miteff F, Butcher K, Lin L, Levi C, et al. Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol. 2017; 82:995–1003.
Article20. Bivard A, Levi C, Spratt N, Parsons M. Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology. 2013; 267:543–550.
Article21. Fahmi F, Marquering HA, Borst J, Streekstra GJ, Beenen LF, Niesten JM, et al. 3D movement correction of CT brain perfusion image data of patients with acute ischemic stroke. Neuroradiology. 2014; 56:445–452.
Article22. Menon BK, Sajobi TT, Zhang Y, Rempel JL, Shuaib A, Thornton J, et al. Analysis of workflow and time to treatment on thrombectomy outcome in the endovascular treatment for small core and proximal occlusion ischemic stroke (ESCAPE) randomized, controlled trial. Circulation. 2016; 133:2279–2286.
Article23. Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020; 395:878–887.24. Stewart EE, Chen X, Hadway J, Lee TY. Correlation between hepatic tumor blood flow and glucose utilization in a rabbit liver tumor model. Radiology. 2006; 239:740–750.
Article25. Johnson JA, Wilson TA. A model for capillary exchange. Am J Physiol. 1966; 210:1299–1303.
Article26. Boutelier T, Kudo K, Pautot F, Sasaki M. Bayesian hemodynamic parameter estimation by bolus tracking perfusion weighted imaging. IEEE Trans Med Imaging. 2012; 31:1381–1395.
Article27. Meijs M, Christensen S, Lansberg MG, Albers GW, Calamante F. Analysis of perfusion MRI in stroke: to deconvolve, or not to deconvolve. Magn Reson Med. 2016; 76:1282–1290.
Article28. McKinley R, Hung F, Wiest R, Liebeskind DS, Scalzo F. A machine learning approach to perfusion imaging with dynamic susceptibility contrast MR. Front Neurol. 2018; 9:717.
Article29. Shen D, Wu G, Suk HI. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017; 19:221–248.
Article30. Hoving JW, Marquering HA, Majoie CBLM, Yassi N, Sharma G, Liebeskind DS, et al. Volumetric and spatial accuracy of computed tomography perfusion estimated ischemic core volume in patients with acute ischemic stroke. Stroke. 2018; 49:2368–2375.
Article31. Qiu W, Kuang H, Teleg E, Ospel JM, Sohn SI, Almekhlafi M, et al. Machine learning for detecting early infarction in acute stroke with non-contrast-enhanced CT. Radiology. 2020; 294:638–644.32. Goyal M, Ospel JM, Menon B, Almekhlafi M, Jayaraman M, Fiehler J, et al. Challenging the ischemic core concept in acute ischemic stroke imaging. Stroke. 2020; 51:3147–3155.
Article33. Kemmling A, Flottmann F, Forkert ND, Minnerup J, Heindel W, Thomalla G, et al. Multivariate dynamic prediction of ischemic infarction and tissue salvage as a function of time and degree of recanalization. J Cereb Blood Flow Metab. 2015; 35:1397–1405.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Machine Learning and Deep Learning in Imaging of Ischemic Stroke
- Characteristics of the Early Electrocardiographic Findings and Their Relations h i p with the Location of the Lesion and Its Severity in Acute Ischemic Stroke
- Application of Artificial Intelligence in Acute Ischemic Stroke: A Scoping Review
- Imaging in Acute Anterior Circulation Ischemic Stroke: Current and Future
- Antiplatelet Therapy for Secondary Stroke Prevention in Patients with Ischemic Stroke or Transient Ischemic Attack