J Korean Med Sci.
2021 May;36(20):e143. 10.3346/jkms.2021.36.e143.
Uptake of Biosimilars and Its Economic Implication for the Treatment of Patients with Rheumatoid Arthritis in Korea
- Affiliations
-
- 1Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea
- 2College of Pharmacy, Chung-Ang University, Seoul, Korea
- KMID: 2516051
- DOI: http://doi.org/10.3346/jkms.2021.36.e143
Abstract
- Background
We aimed to examine the uptake of infliximab and etanercept biosimilars in patients with rheumatoid arthritis (RA) and its economic implication for healthcare expenditure.
Methods
Using Korean Health Insurance Review and Assessment Service National Patient Samples, we extracted RA patients who used biologic disease modifying anti-rheumatic drugs (bDMARDs) between 2009 and 2018. Descriptive statistics were used to explain the basic features of the data. We calculated the proportion of users of each bDMARD among total patients with bDMARDs half-yearly. We assessed changes in the utilization proportions of bDMARDs including 4 tumor necrosis factor inhibitors (TNFis) and 2 non-TNFis, which have been approved for RA in Korea: etanercept, infliximab, adalimumab, golimumab, tocilizumab, and abatacept, and analyzed the changes in market share of biosimilars among the bDMARDs after their introduction. Overall trends of medical costs for each bDMARD were presented over the 10-year period.
Results
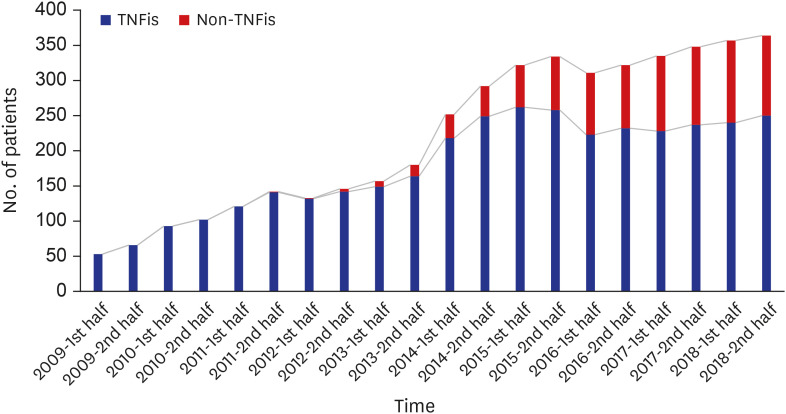
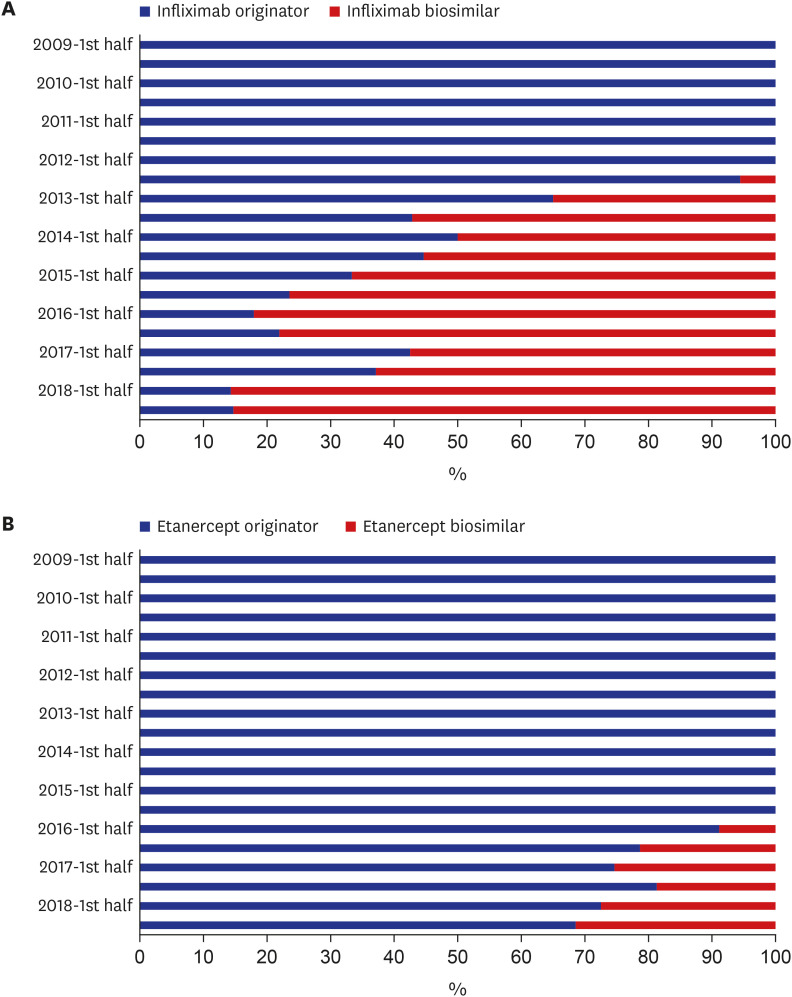
Since the introduction of the biosimilar TNFis in 2012, the proportion of their use among bDMARDs steadily increased to 15.8% in 2018. While there has been a gradual increase in the use of biosimilar TNFis, the use of the corresponding originators has been decreasing. The introduction of biosimilar TNFis has resulted in a decrease in the medical costs of patients using either originator or biosimilar TNFis.
Conclusion
In Korea, the proportional use of biosimilar TNFis has gradually increased since their introduction. The availability of less expensive biosimilar TNFis seems to have brought about a decrease in the medical costs of users of the originators.
Keyword
Figure
Reference
-
1. Schulze-Koops H, Skapenko A. Biosimilars in rheumatology: a review of the evidence and their place in the treatment algorithm. Rheumatology (Oxford). 2017; 56(suppl_4):iv30–iv48. PMID: 28903543.
Article2. Scheinberg MA, Kay J. The advent of biosimilar therapies in rheumatology--“O brave new world”. Nat Rev Rheumatol. 2012; 8(7):430–436. PMID: 22664834.
Article3. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017; 76(6):960–977. PMID: 28264816.4. Improving access to biosimilars in low-income countries. Lancet. 2017; 389(10082):1860.5. QuintilesIMS. The impact of biosimilar competition in Europe. Updated 2017. Accessed April 15, 2021. http://ec.europa.eu/growth/content/impact-biosimilar-competition-price-volume-and-market-share-update-2017-0_en.6. Brodszky V, Gulacsi L, Balogh O, Baji P, Rencz F, Péntek M. Budget impact analysis of biosimilar infliximab for the treatment of Crohn's disease in six central eastern European countries. Value Health. 2014; 17(7):A364.
Article7. Kim SC, Sarpatwari A, Landon JE, Desai RJ. Utilization and treatment costs of tumor necrosis factor inhibitors after the introduction of biosimilar infliximab in the United States. Arthritis Rheumatol. 2020; 72(6):1036–1038. PMID: 31943866.
Article8. Bansback N, Curtis JR, Huang J, He Z, Evans M, Johansson T, et al. Patterns of tumor necrosis factor inhibitor (TNFi) biosimilar use across United States rheumatology practices. ACR Open Rheumatol. 2020; 2(2):79–83. PMID: 32043831.9. Hakim A, Ross JS. Obstacles to the adoption of biosimilars for chronic diseases. JAMA. 2017; 317(21):2163–2164. PMID: 28459924.
Article10. Park EJ, Kim H, Jung SM, Sung YK, Baek HJ, Lee J. The use of biological disease-modifying antirheumatic drugs for inflammatory arthritis in Korea: results of a Korean Expert Consensus. Korean J Intern Med. 2020; 35(1):41–59. PMID: 31935319.
Article11. Kim J, Ha D, Song I, Park H, Lee SW, Lee EK, et al. Estimation of cost savings between 2011 and 2014 attributed to infliximab biosimilar in the South Korean healthcare market: real-world evidence using a nationwide database. Int J Rheum Dis. 2018; 21(6):1227–1236. PMID: 29667324.
Article12. Sung YK, Jung SY, Kim H, Choi S, Im SG, Lee YS, et al. Factors for starting biosimilar TNF inhibitors in patients with rheumatic diseases in the real world. PLoS One. 2020; 15(1):e0227960. PMID: 31978121.
Article13. Kim S, Kim MS, You SH, Jung SY. Conducting and reporting a clinical research using Korean healthcare claims database. Korean J Fam Med. 2020; 41(3):146–152. PMID: 32456382.
Article14. Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health. 2014; 36:e2014008. PMID: 25078381.
Article15. Jensen TB, Bartels D, Sædder EA, Poulsen BK, Andersen SE, Christensen MM, et al. The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol. 2020; 76(1):35–40. PMID: 31677117.
Article16. Jensen TB, Kim SC, Jimenez-Solem E, Bartels D, Christensen HR, Andersen JT. Shift from adalimumab originator to biosimilars in Denmark. JAMA Intern Med. 2020; 180(6):902–903. PMID: 32227137.
Article17. Aladul MI, Fitzpatrick RW, Chapman SR. Impact of infliximab and etanercept biosimilars on biological disease-modifying antirheumatic drugs utilisation and NHS Budget in the UK. BioDrugs. 2017; 31(6):533–544. PMID: 29127626.
Article18. Kim H, Alten R, Avedano L, Dignass A, Gomollón F, Greveson K, et al. The future of biosimilars: maximizing benefits across immune-mediated inflammatory diseases. Drugs. 2020; 80(2):99–113. PMID: 32002851.
Article19. Pouillon L, Socha M, Demore B, Thilly N, Abitbol V, Danese S, et al. The nocebo effect: a clinical challenge in the era of biosimilars. Expert Rev Clin Immunol. 2018; 14(9):739–749. PMID: 30118338.
Article20. Kim HA, Lee E, Lee SK, Park YB, Lee YN, Kang HJ, et al. Retention rate and safety of biosimilar CT-P13 in rheumatoid arthritis: data from the Korean College of Rheumatology Biologics Registry. BioDrugs. 2020; 34(1):89–98. PMID: 31734899.
Article