J Korean Neurosurg Soc.
2021 May;64(3):329-339. 10.3340/jkns.2020.0125.
Gastrulation : Current Concepts and Implications for Spinal Malformations
- Affiliations
-
- 1Department of Paediatric Neurosurgery, Great Ormond Street Hospital for Children NHS Trust, London, UK
- KMID: 2515488
- DOI: http://doi.org/10.3340/jkns.2020.0125
Abstract
- It has been recognised for over a century that the events of gastrulation are fundamental in determining, not only the development of the neuraxis but the organisation of the entire primitive embryo. Until recently our understanding of gastrulation was based on detailed histological analysis in animal models and relatively rare human tissue preparations from aborted fetuses. Such studies resulted in a model of gastrulation that neurosurgeons have subsequently used as a means of trying to explain some of the congenital anomalies of caudal spinal cord and vertebral development that present in paediatric neurosurgical practice. Recent advances in developmental biology, in particular cellular biology and molecular genetics have offered new insights into very early development. Understanding the processes that underlie cellular interactions, gene expression and activation/inhibition of signalling pathways has changed the way embryologists view gastrulation and this has led to a shift in emphasis from the ‘descriptive and morphological’ to the ‘mechanistic and functional’. Unfortunately, thus far it has proved difficult to translate this improved knowledge of normal development, typically derived from non-human models, into an understanding of the mechanisms underlying human malformations such as the spinal dysraphisms and anomalies of caudal development. A paediatric neurosurgeons perspective of current concepts in gastrulation is presented along with a critical review of the current hypotheses of human malformations that have been attributed to disorders of this stage of embryogenesis.
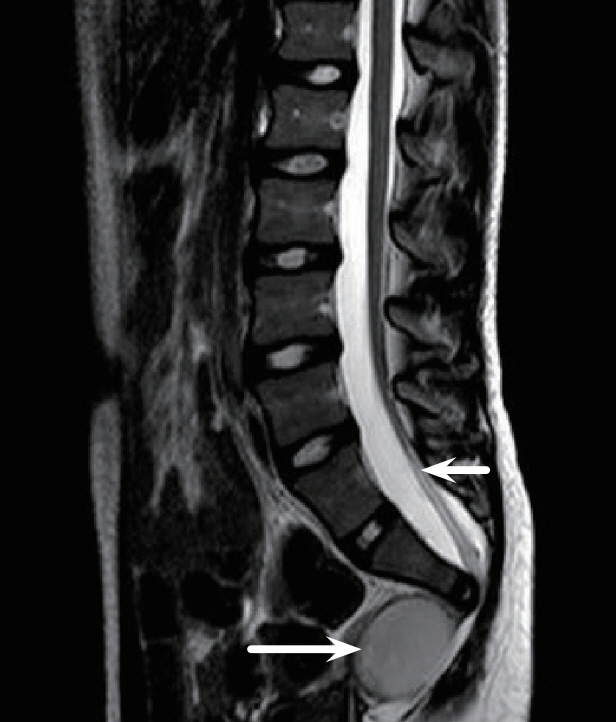
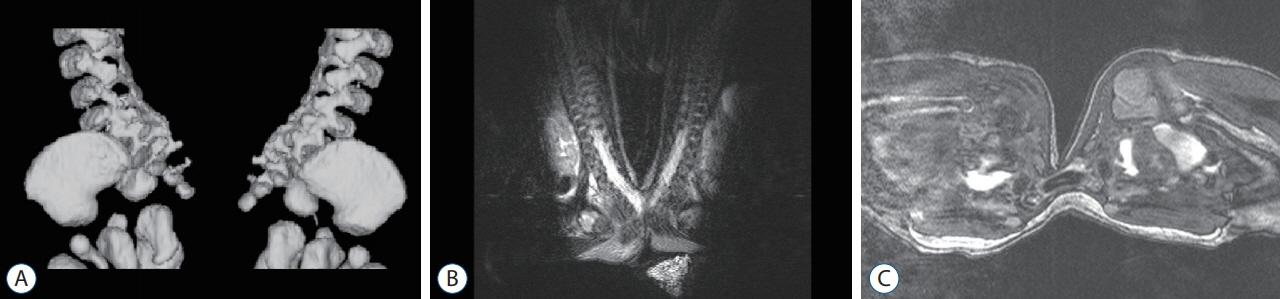
Figure
Reference
-
References
1. Bremer JL. Dorsal intestinal fistula; accessory neurenteric canal; diastematomyelia. AMA Arch Pathol. 54:132–138. 1952.2. Cearns MD, Hettige S, De Coppi P, Thompson DNP. Currarino syndrome: repair of the dysraphic anomalies and resection of the presacral mass in a combined neurosurgical and general surgical approach. J Neurosurg Pediatr. 22:584–590. 2018.
Article3. Corallo D, Trapani V, Bonaldo P. The notochord: structure and functions. Cell Mol Life Sci. 72:2989–3008. 2015.
Article4. Dias LA, Nakanishi M, Mangussi-Gomes J, Canuto M, Takano G, Oliveira CA. Successful endoscopic endonasal management of a transclival cerebrospinal fluid fistula secondary to ecchordosis physaliphora--an ectopic remnant of primitive notochord tissue in the clivus. Clin Neurol Neurosurg. 117:116–119. 2014.
Article5. Dias MS, Azizkhan RG. A novel embryogenetic mechanism for Currarino’s triad: inadequate dorsoventral separation of the caudal eminence from hindgut endoderm. Pediatr Neurosurg. 28:223–229. 1998.
Article6. Emura T, Asashima M, Furue M, Hashizume K. Experimental split cord malformations. Pediatr Neurosurg. 36:229–235. 2002.
Article7. Emura T, Asashima M, Hashizume K. An experimental animal model of split cord malformation. Pediatr Neurosurg. 33:283–292. 2000.
Article8. Escobar LF, Heiman M, Zimmer D, Careskey H. Urorectal septum malformation sequence: prenatal progression, clinical report, and embryology review. Am J Med Genet A. 143A:2722–2726. 2007.
Article9. Escobar LF, Weaver DD, Bixler D, Hodes ME, Mitchell M. Urorectal septum malformation sequence. Report of six cases and embryological analysis. Am J Dis Child. 141:1021–1024. 1987.10. Ferrer-Vaquer A, Hadjantonakis AK. Birth defects associated with perturbations in preimplantation, gastrulation, and axis extension: from conjoined twinning to caudal dysgenesis. Wiley Interdiscip Rev Dev Biol. 2:427–442. 2013.
Article11. Ferrer-Vaquer A, Viotti M, Hadjantonakis AK. Transitions between epithelial and mesenchymal states and the morphogenesis of the early mouse embryo. Cell Adh Migr. 4:447–457. 2010.
Article12. Fieggen AG, Dunn RN, Pitcher RD, Millar AJ, Rode H, Peter JC. Ischiopagus and pygopagus conjoined twins: neurosurgical considerations. Childs Nerv Syst. 20:640–651. 2004.
Article13. Gegg CA, Vollmer DG, Tullous MW, Kagan-Hallet KS. An unusual case of the complete Currarino triad: case report, discussion of the literature and the embryogenic implications. Neurosurgery. 44:658–662. 1999.
Article14. Jo Mauch T, Albertine KH. Urorectal septum malformation sequence: Insights into pathogenesis. Anat Rec. 268:405–410. 2002.
Article15. Köchling J, Karbasiyan M, Reis A. Spectrum of mutations and genotypephenotype analysis in Currarino syndrome. Eur J Hum Genet. 9:599–605. 2001.
Article16. Kole MJ, Fridley JS, Jea A, Bollo RJ. Currarino syndrome and spinal dysraphism. J Neurosurg Pediatr. 13:685–689. 2014.
Article17. Lagman C, Varshneya K, Sarmiento JM, Turtz AR, Chitale RV. Proposed diagnostic criteria, classification schema, and review of literature of notochord-derived ecchordosis physaliphora. Cureus. 8:e547. 2016.
Article18. Menezes AH, Traynelis VC. Spinal neurenteric cysts in the magnetic resonance imaging era. Neurosurgery. 58:97–105. discussion 97-105. 2006.
Article19. Padmanabhan R. Retinoic acid-induced caudal regression syndrome in the mouse fetus. Reprod Toxicol. 12:139–151. 1998.
Article20. Paleologos TS, Thom M, Thomas DG. Spinal neurenteric cysts without associated malformations. Are they the same as those presenting in spinal dysraphism? Br J Neurosurg. 14:185–194. 2000.21. Pang D, Dias MS, Ahab-Barmada M. Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 31:451–480. 1992.22. Pennimpede T, Proske J, König A, Vidigal JA, Morkel M, Bramsen JB, et al. In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev Biol. 372:55–67. 2012.
Article23. Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 136:701–713. 2009.
Article24. Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 20:986–997. 2009.
Article25. Rulle A, Tsikolia N, de Bakker B, Drummer C, Behr R, Viebahn C. On the enigma of the human neurenteric canal. Cells Tissues Organs. 205:256–278. 2018.
Article26. Sayyid SK, Wong PK, Read W, Monson DK, Umpierrez M, Gonzalez F, et al. The clincoradiologic spectrum of notochordal derived masses. Clin Imaging. 56:124–134. 2019.
Article27. Sekiya K, Watanabe M, Nadgir RN, Buch K, Flower EN, Kaneda T, et al. Nasopharyngeal cystic lesions: Tornwaldt and mucous retention cysts of the nasopharynx: findings on MR imaging. J Comput Assist Tomogr. 38:9–13. 2014.28. Shukla M, Behari S, Guruprasad B, Das KK, Mehrotra A, Srivastava AK, et al. Spinal neurenteric cysts: associated developmental anomalies and rationale of surgical approaches. Acta Neurochir (Wien). 157:1601–1610. 2015.
Article29. Thottungal AD, Charles AK, Dickinson JE, Bower C. Caudal dysgenesis and sirenomelia-single centre experience suggests common pathogenic basis. Am J Med Genet A. 152A:2578–2587. 2010.
Article30. Vaishya S, Kumarjain P. Split cord malformation: three unusual cases of composite split cord malformation. Childs Nerv Syst. 17:528–530. 2001.
Article31. Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 449:1049–1052. 2007.
Article32. Wolpert L. The triumph of the embryo. Oxford: Oxford University Press;1991.33. Xanthos JB, Kofron M, Tao Q, Schaible K, Wylie C, Heasman J. The roles of three signaling pathways in the formation and function of the Spemann Organizer. Development. 129:4027–4043. 2002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Understanding of Human Embryo Development for Teratogen Counselling
- A Case of Giant Ependymomas of the Cauda Equina associated with Thoracic Intradural Spinal A-V Malformations
- Intervention of Vascular Diseases of the Spine
- A Review of the Current State and Future Directions for Management of Scalp and Facial Vascular Malformations
- Pediatric Central Nervous System Vascular Malformation : Pathological Review with Diagram