J Liver Cancer.
2021 Mar;21(1):97-103. 10.17998/jlc.21.1.97.
Advanced Stage Hepatocellular Carcinoma Successfully Treated with Liver-directed Concurrent Chemoradiotherapy and Sequential Transarterial Radio-embolization
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
- 3Yonsei Liver Center, Severance Hospital, Seoul, Korea
- KMID: 2514241
- DOI: http://doi.org/10.17998/jlc.21.1.97
Abstract
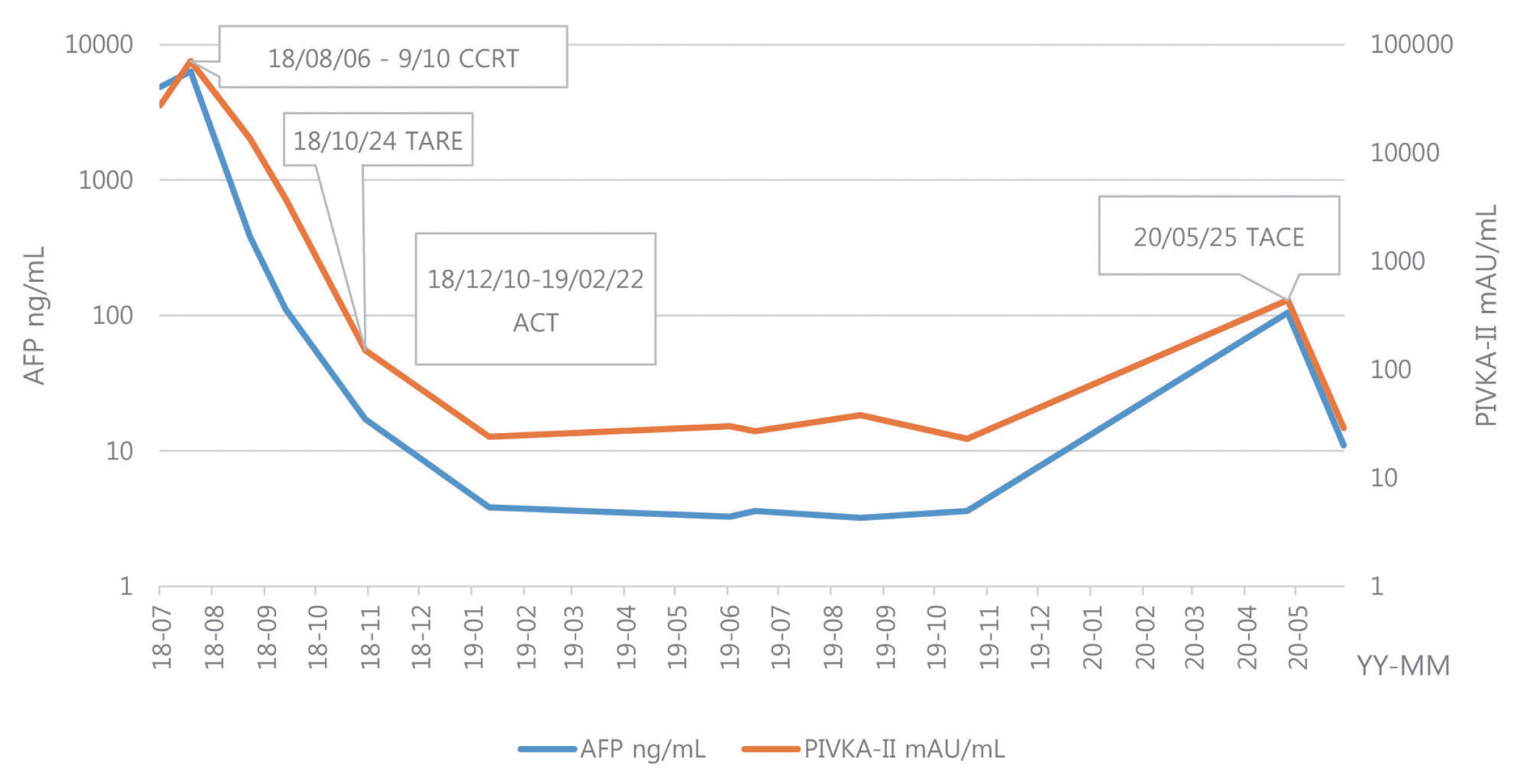
- Optimal treatment strategies for patients with advanced hepatocellular carcinoma (HCC) is yet to be determined. Herein, we present a case of advanced HCC with tumor invasion into the right anterior portal vein and right hepatic vein where complete response (CR) was achieved via a multidisciplinary approach. This patient had a 10.5 cm-sized HCC invading segment VI, without extrahepatic spread. Liver function was classified as Child-Pugh class A, and the performance status was good. Transarterial radio-embolization (TARE) was performed 6 weeks after the completion of liver-directed concurrent chemoradiotherapy, and CR was confirmed 3 months post-TARE. Adoptive cell therapies were performed as adjuvant therapy and CR was maintained for over 15 months, until the local recurrence of a 2 cm-sized HCC was found. Therefore, in selected cases with preserved liver function, combination therapies, including LRTs and systemic therapy, can be a useful therapeutic option for advanced HCC.
Figure
Reference
-
1. Chan SL, Chong CCN, Chan AWH, Poon DMC, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol. 2016; 22:7289–7300.2. Díaz-González Á, Reig M, Bruix J. Treatment of hepatocellular carcinoma. Dig Dis. 2016; 34:597–602.3. Moriguchi M, Aramaki T, Nishiofuku H, Sato R, Asakura K, Yamaguchi K, et al. Sorafenib versus hepatic arterial infusion chemotherapy as initial treatment for hepatocellular carcinoma with advanced portal vein tumor thrombosis. Liver Cancer. 2017; 6:275–286.4. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669.5. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017; 66:545–551.6. Colagrande S, Inghilesi AL, Aburas S, Taliani GG, Nardi C, Marra F. Challenges of advanced hepatocellular carcinoma. World J Gastroenterol. 2016; 22:7645–7659.7. Kim BK, Kim DY, Byun HK, Choi HJ, Beom SH, Lee HW, et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2020; 107:106–115.8. Han S, Lee HW, Park JY, Kim SU, Kim DY, Ahn SH, et al. Appraisal of long-term outcomes of liver-directed concurrent chemoradiotherapy for hepatocellular carcinoma with major portal vein invasion. J Hepatocell Carcinoma. 2020; 7:403–412.9. Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018; 68:1429–1440.10. Sangro B, Gardini AC. Radioembolisation in hepatocellular carcinoma: principles of management. Cross T, Palmer DH, editors. Liver cancers: from mechanisms to management. Cham: Springer International Publishing;2019. p. 139–152.11. Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005; 140:450–457. discussion 457–458.12. Hermanek P, Hendson DE, Hutter RVP, Sobin LH. TNM supplement 1993: a commentary on uniform use. Heidelberg: Springer-Verlag;1993. p. 1–142.13. Edeline J, Crouzet L, Campillo-Gimenez B, Rolland Y, Pracht M, Guillygomarc’h A, et al. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2016; 43:635–643.14. Schiro BJ, Amour ES, Harnain C, Gandhi RT. Management of high hepatopulmonary shunts in the setting of Y90 radioembolization. Tech Vasc Interv Radiol. 2019; 22:58–62.15. Theysohn JM, Schlaak JF, Müller S, Ertle J, Schlosser TW, Bockisch A, et al. Selective internal radiation therapy of hepatocellular carcinoma: potential hepatopulmonary shunt reduction after sorafenib administration. J Vasc Interv Radiol. 2012; 23:949–952.16. Cha H, Yoon HI, Lee IJ, Koom WS, Han KH, Seong J. Clinical factors related to recurrence after hepatic arterial concurrent chemoradiotherapy for advanced but liver-confined hepatocellular carcinoma. J Radiat Res. 2013; 54:1069–1077.17. Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90Yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995; 33:919–924.18. Mokdad AA, Singal AG, Yopp AC. Advances in local and systemic therapies for hepatocellular cancer. Curr Oncol Rep. 2016; 18:9.19. Hendrickson PG, Olson M, Luetkens T, Weston S, Han T, Atanackovic D, et al. The promise of adoptive cellular immunotherapies in hepatocellular carcinoma. Oncoimmunology. 2020; 9:1673129.20. Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000; 356:802–807.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term survival after CCRT and HAIC followed by ALPPS for hepatocellular carcinoma with portal vein invasion: a case report
- A Case of Concurrent Chemoradiation Therapy for Locally Advanced Hepatocellular Carcinoma with Portal Vein Thrombosis
- A Case of Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis Treated by Hepatic Artery Injection Chemotherapy and Radiotherapy
- Complete Response of Single Nodular Large Hepatocellular Carcinoma with Pulmonary Metastasis by Sequential Transarterial Chemoembolization and Sorafenib: A Case Report
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report