Obstet Gynecol Sci.
2021 Mar;64(2):201-208. 10.5468/ogs.20247.
Non-compliance with pregnancy prevention recommendations for isotretinoin in Korea between 2019-2020
- Affiliations
-
- 1Korean MotherSafe Counselling Center, Pregnancy & Breastfeeding Medicines Information Center, Seoul, Korea
- 2Department of Obstetrics and Gynaecology, Hanmaeum Changwon Hospital, Changwon, Korea
- 3Department of Obstetrics and Gynaecology, Inje University, Ilsan Paik Hospital, Goyang, Korea
- KMID: 2513949
- DOI: http://doi.org/10.5468/ogs.20247
Abstract
Objective
Isotretinoin is commonly prescribed worldwide despite its notorious teratogenicity. A risk management program (RMP) was introduced in Korea to prevent isotretinoin use during pregnancy. Here, we evaluate the compliance of Korean women with the recommendations of the RMP.
Methods
This prospective cohort study was conducted between April 2019 and June 2020. Thirty-six and 82 patients received the prescription before and after the introduction of RMP, respectively.
Results
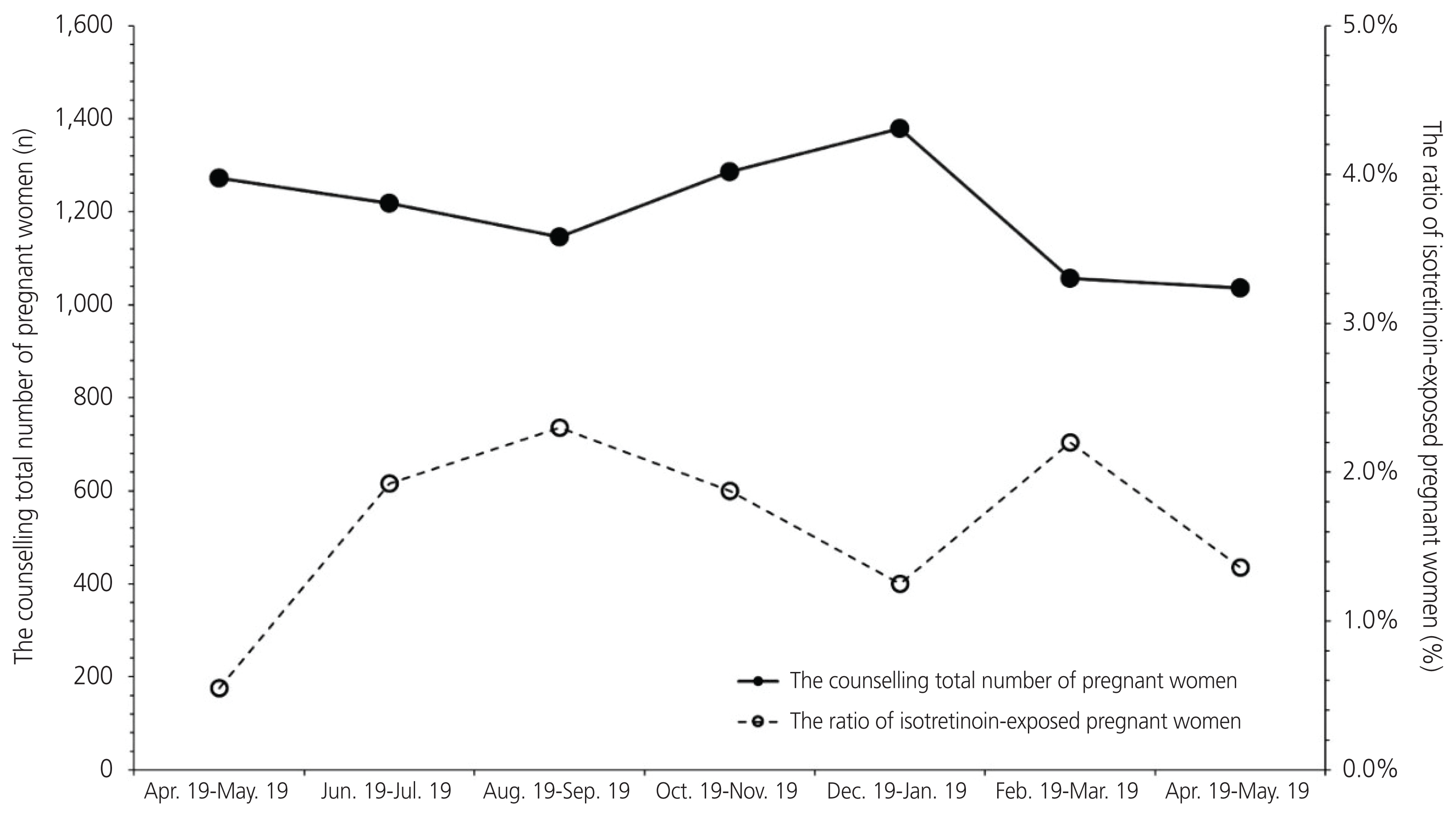
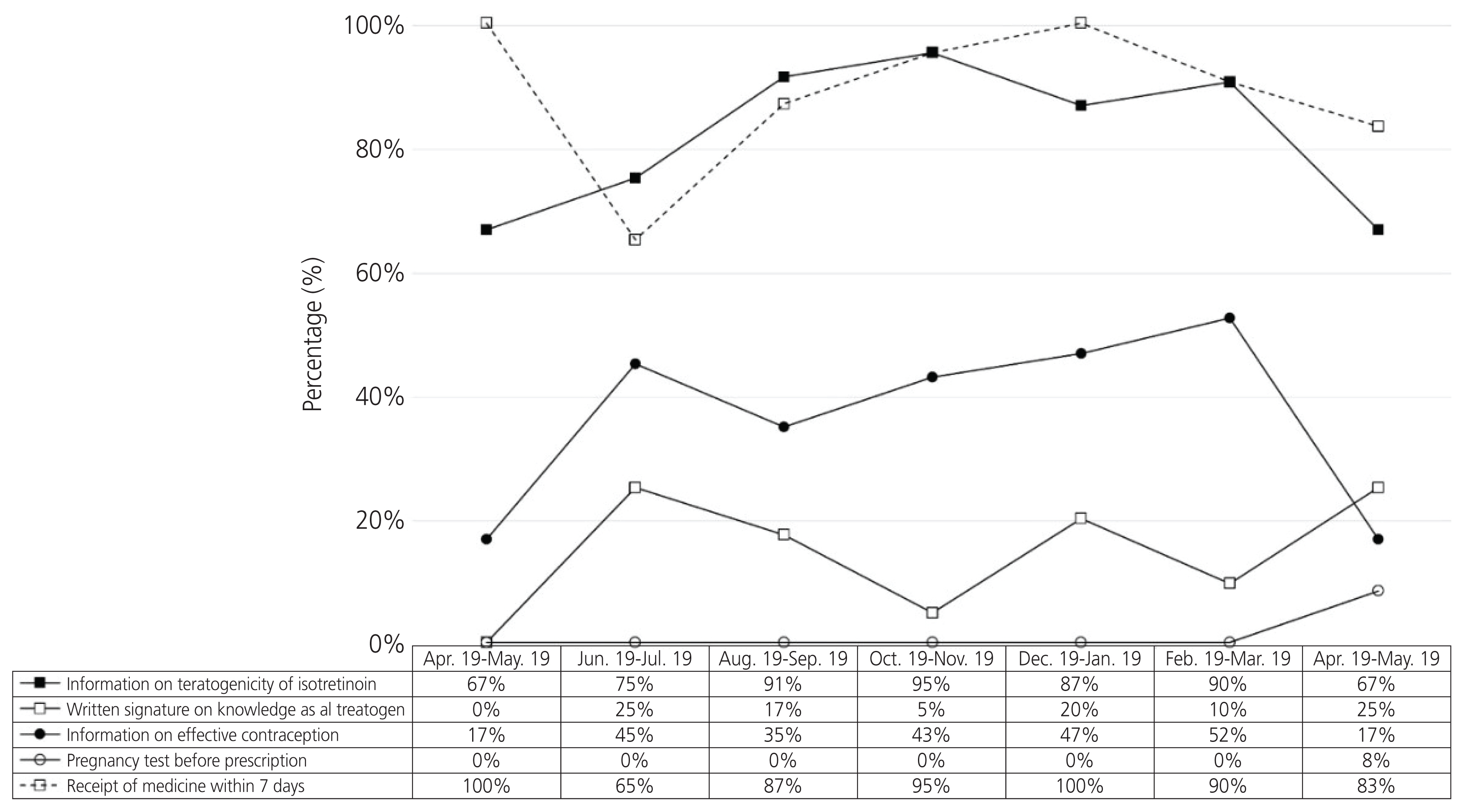
There was a significant difference in the total number of days for which isotretinoin was prescribed before and after the RMP was introduced (68.8±100.9 and 28.0±26.1 days, respectively). However, 1.43% (120/8,394) of the total patients contacted by the teratology information services were exposed to isotretinoin on an average.
Conclusion
The proportion of patients exposed to isotretinoin did not change, and there was no significant change in compliance, with the implementation of the RMP during the study period. Further studies are needed to evaluate the effectiveness of the RMP in the long term.
Figure
Cited by 1 articles
-
Pregnancy and neonatal outcomes after periconceptional exposure to isotretinoin in Koreans
Eun-Hwan Cha, NaeRy Kim, Ho-Seok Kwak, Hae Ji Han, Sung Hong Joo, June-Seek Choi, Kyoung-Chul Chun, Young-Ah Kim, Jae-Whoan Koh, Jung Yeol Han
Obstet Gynecol Sci. 2022;65(2):166-175. doi: 10.5468/ogs.21354.
Reference
-
References
1. Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009; 1:162–9.
Article2. Honein MA, Paulozzi LJ, Erickson JD. Continued occurrence of accutane-exposed pregnancies. Teratology. 2001; 64:142–7.
Article3. Azoulay L, Oraichi D, Bérard A. Patterns and utilization of isotretinoin for acne from 1984 to 2003: is there need for concern? Eur J Clin Pharmacol. 2006; 62:667–74.
Article4. Kim NR, Yoon SR, Choi JS, Ahn HK, Lee SY, Hong DS, et al. Isotretinoin exposure in pregnant women in Korea. Obstet Gynecol Sci. 2018; 61:649–54.
Article5. Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. N Engl J Med. 1985; 313:837–41.
Article6. Bérard A, Azoulay L, Koren G, Blais L, Perreault S, Oraichi D. Isotretinoin, pregnancies, abortions and birth defects: a population-based perspective. Br J Clin Pharmacol. 2007; 63:196–205.
Article7. Mitchell AA, Van Bennekom CM, Louik C. A pregnancy-prevention program in women of childbearing age receiving isotretinoin. N Engl J Med. 1995; 333:101–6.
Article8. Schwetz BA. From the food and drug administration. JAMA. 2002; 287:578.9. Abroms L, Maibach E, Lyon-Daniel K, Feldman SR. What is the best approach to reducing birth defects associated with isotretinoin? PLoS Med. 2006; 3:e483.
Article10. Kovitwanichkanont T, Driscoll T. A comparative review of the isotretinoin pregnancy risk management programs across four continents. Int J Dermatol. 2018; 57:1035–46. https://doi.org/10.1111/ijd.13950 PubMed.
Article11. iPLEDGE. Committed to pregnancy prevention [internet]. White Oak (MA): iPLEDGE;2016. [cited 2020 Jul 14]. Available from: https://www.ipledgeprogram.com/iPledgeUI/home.u .12. Yook JH, Han JY, Choi JS, Ahn HK, Lee SW, Kim MY, et al. Pregnancy outcomes and factors associated with voluntary pregnancy termination in women who had been treated for acne with isotretinoin. Clin Toxicol (Phila). 2012; 50:896–901.
Article13. Garcia-Bournissen F, Tsur L, Goldstein LH, Staroselsky A, Avner M, Asrar F, et al. Fetal exposure to isotretinoin-an international problem. Reprod Toxicol. 2008; 25:124–8.
Article14. Crijns HJ, Straus SM, Gispen-de Wied C, de Jong-van den Berg LT. Compliance with pregnancy prevention programmes of isotretinoin in Europe: a systematic review. Br J Dermatol. 2011; 164:238–44.
Article15. Uusküla A, Pisarev H, Kurvits K, Laius O, Laanpere M, Uusküla M. Compliance with pregnancy prevention recommendations for isotretinoin in Estonia in 2012–2016. Drugs Real World Outcomes. 2018; 5:129–36.
Article16. Shin J, Cheetham TC, Wong L, Niu F, Kass E, Yoshinaga MA, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011; 65:1117–25.
Article17. Pinheiro SP, Kang EM, Kim CY, Governale LA, Zhou EH, Hammad TA. Concomitant use of isotretinoin and contraceptives before and after iPLEDGE in the United States. Pharmacoepidemiol Drug Saf. 2013; 22:1251–7.
Article18. Charrow A, Xia FD, Lu J, Waul M, Joyce C, Mostaghimi A. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PLoS One. 2019; 14:e0210445.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Impetigo Herpetiformis Treated with Isotretinoin
- Pregnant Women's Knowledge and Compliance about Prevention of Respiratory Infection
- A Case of Confluent and Reticulated Papillomatosis Treated with Oral Isotretinoin
- Isotretinoin exposure in pregnant women in Korea
- Sebaceous Hyperplasia Treated with Isotretinoin