Int J Stem Cells.
2021 Feb;14(1):119-126. 10.15283/ijsc20152.
Production of Functional Hepatobiliary Organoids from Human Pluripotent Stem Cells
- Affiliations
-
- 1Department of Central Laboratory, Shenzhen Hospital, Beijing University of Chinese Medicine, Shenzhen, China
- 2Department of Hepatology, Shenzhen Hospital, Beijing University of Chinese Medicine, Shenzhen, China
- KMID: 2513089
- DOI: http://doi.org/10.15283/ijsc20152
Abstract
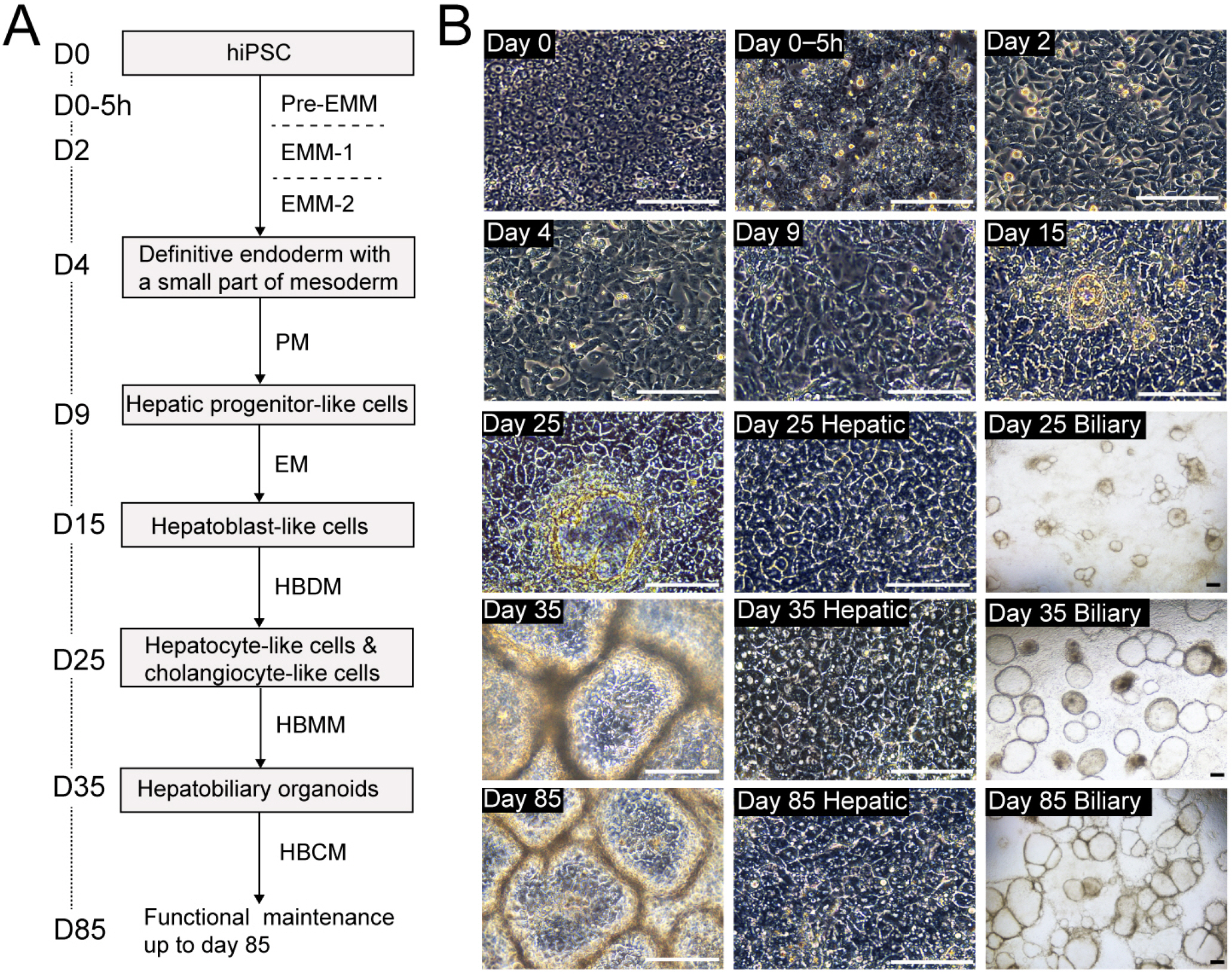
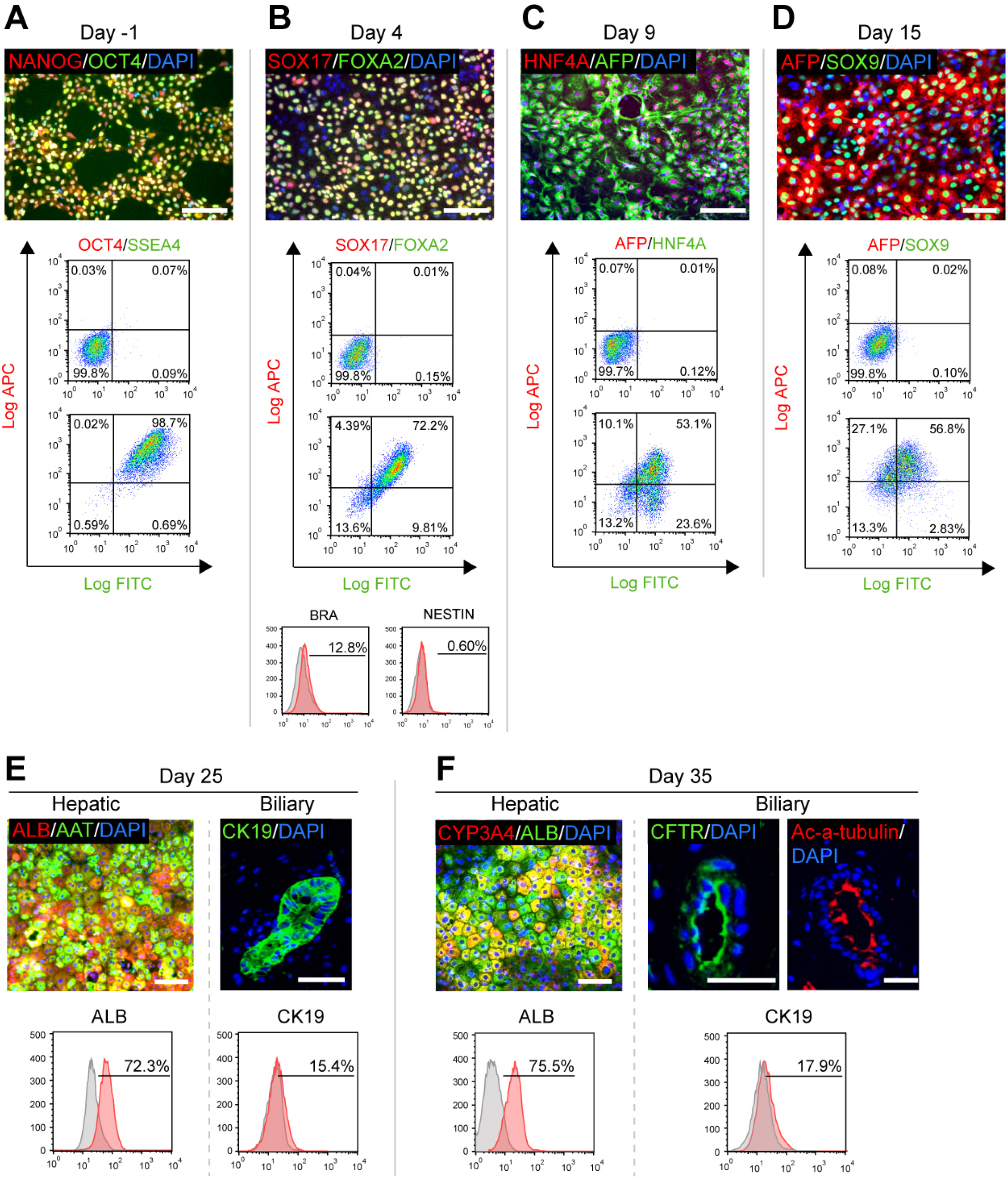
- The research on human hepatobiliary development and disorders has been constrained by minimal access to human fetal tissue, and low accuracy of animal models. To overcome this problem, we have established a system for the differentiation of human pluripotent stem cells (hPSCs) into functional hepatobiliary organoids (HBOs). We have previously reported that our 45-d approach closely mimics key stages of hepatobiliary development, starting with the differentiation of hiPSC into endoderm and a small part of mesoderm, and subsequently into hepatoblast-like cells, followed by the parallel generation of hepatocyte-like cells and cholangiocyte-like cells, formation of immature HBO expressing early hepatic and biliary markers, and mature HBO displaying hepatobiliary functionality. In this study, we present an updated version of our previous protocol, which only needs 35 days to achieve maturation in vitro. Furthermore, a hepatobiliary culture medium is developed to functionally maintain the HBOs for more than 1.5 months. The capacity of this approach for producing large amounts of functional HBOs and enabling long-term culture in vitro holds promise for applications on developmental research, disease modeling, as well as screening of therapeutic agents.
Figure
Reference
-
References
1. Trounson A, DeWitt ND. 2016; Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 17:194–200. DOI: 10.1038/nrm.2016.10. PMID: 26908143.
Article2. Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. 2007; Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 45:1229–1239. DOI: 10.1002/hep.21582. PMID: 17464996.
Article3. Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H, Yin X, Wu C, Che J, Lu S, Ding M, Deng H. 2009; Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 19:1233–1242. DOI: 10.1038/cr.2009.107. PMID: 19736565.
Article4. Zhao D, Chen S, Duo S, Xiang C, Jia J, Guo M, Lai W, Lu S, Deng H. 2013; Promotion of the efficient metabolic maturation of human pluripotent stem cell-derived hepatocytes by correcting specification defects. Cell Res. 23:157–161. DOI: 10.1038/cr.2012.144. PMID: 23070301. PMCID: PMC3541654.
Article5. Noto FK, Duncan SA. Sell S, editor. 2013. Generation of hepatocyte-like cells from human pluripotent stem cells. Stem Cells Handbook. Humana Press;New York: p. 139–147. DOI: 10.1007/978-1-4614-7696-2_10.
Article6. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. 2013; Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499:481–484. DOI: 10.1038/nature12271. PMID: 23823721.
Article7. Guye P, Ebrahimkhani MR, Kipniss N, Velazquez JJ, Schoenfeld E, Kiani S, Griffith LG, Weiss R. 2016; Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat Commun. 7:10243. DOI: 10.1038/ncomms10243. PMID: 26732624. PMCID: PMC4729822.
Article8. Duncan AW, Dorrell C, Grompe M. 2009; Stem cells and liver regeneration. Gastroenterology. 137:466–481. DOI: 10.1053/j.gastro.2009.05.044. PMID: 19470389. PMCID: PMC3136245.
Article9. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H. 2015; Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 160:299–312. DOI: 10.1016/j.cell.2014.11.050. PMID: 25533785. PMCID: PMC4313365.
Article10. Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. 2011; Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 43:245–256. DOI: 10.1016/j.biocel.2009.07.020. PMID: 19735739.
Article11. Sherlock S, Dooley JS. 2002. Diseases of the liver and biliary system. 11th ed. Blackwell Science;Oxford: p. 597–623. DOI: 10.1002/9780470986820.12. Dianat N, Dubois-Pot-Schneider H, Steichen C, Desterke C, Leclerc P, Raveux A, Combettes L, Weber A, Corlu A, Dubart-Kupperschmitt A. 2014; Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 60:700–714. DOI: 10.1002/hep.27165. PMID: 24715669. PMCID: PMC4315871.
Article13. Sampaziotis F, de Brito MC, Madrigal P, Bertero A, Saeb-Parsy K, Soares FAC, Schrumpf E, Melum E, Karlsen TH, Bradley JA, Gelson WT, Davies S, Baker A, Kaser A, Alexander GJ, Hannan NRF, Vallier L. 2015; Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 33:845–852. DOI: 10.1038/nbt.3275. PMID: 26167629. PMCID: PMC4768345.
Article14. Ogawa M, Ogawa S, Bear CE, Ahmadi S, Chin S, Li B, Grompe M, Keller G, Kamath BM, Ghanekar A. 2015; Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 33:853–861. DOI: 10.1038/nbt.3294. PMID: 26167630.
Article15. Prior N, Inacio P, Huch M. 2019; Liver organoids: from basic research to therapeutic applications. Gut. 68:2228–2237. DOI: 10.1136/gutjnl-2019-319256. PMID: 31300517. PMCID: PMC6872443.
Article16. Wu F, Wu D, Ren Y, Huang Y, Feng B, Zhao N, Zhang T, Chen X, Chen S, Xu A. 2019; Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 70:1145–1158. DOI: 10.1016/j.jhep.2018.12.028. PMID: 30630011.
Article17. Takayama K, Inamura M, Kawabata K, Sugawara M, Kikuchi K, Higuchi M, Nagamoto Y, Watanabe H, Tashiro K, Sakurai F, Hayakawa T, Furue MK, Mizuguchi H. 2012; Generation of metabolically functioning hepatocytes from human pluripotent stem cells by FOXA2 and HNF1α transduction. J Hepatol. 57:628–636. DOI: 10.1016/j.jhep.2012.04.038. PMID: 22659344.
Article18. Loh KM, Chen A, Koh PW, Deng TZ, Sinha R, Tsai JM, Barkal AA, Shen KY, Jain R, Morganti RM, Shyh-Chang N, Fernhoff NB, George BM, Wernig G, Salomon REA, Chen Z, Vogel H, Epstein JA, Kundaje A, Talbot WS, Beachy PA, Ang LT, Weissman IL. 2016; Mapping the pairwise choices leading from pluripotency to human bone, heart, and other mesoderm cell types. Cell. 166:451–467. DOI: 10.1016/j.cell.2016.06.011. PMID: 27419872. PMCID: PMC5474394.
Article19. Lu J, Zhong X, Liu H, Hao L, Huang CT, Sherafat MA, Jones J, Ayala M, Li L, Zhang SC. 2016; Generation of serotonin neurons from human pluripotent stem cells. Nat Biotechnol. 34:89–94. DOI: 10.1038/nbt.3435. PMID: 26655496. PMCID: PMC4711820.
Article20. Carpentier A, Nimgaonkar I, Chu V, Xia Y, Hu Z, Liang TJ. 2016; Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen. Stem Cell Res. 16:640–650. DOI: 10.1016/j.scr.2016.03.009. PMID: 27062358. PMCID: PMC4903911.
Article21. Touboul T, Chen S, To CC, Mora-Castilla S, Sabatini K, Tukey RH, Laurent LC. 2016; Stage-specific regulation of the WNT/β-catenin pathway enhances differentiation of hESCs into hepatocytes. J Hepatol. 64:1315–1326. DOI: 10.1016/j.jhep.2016.02.028. PMID: 26921690. PMCID: PMC5010388.
Article22. Tian L, Deshmukh A, Ye Z, Jang YY. 2016; Efficient and controlled generation of 2D and 3D bile duct tissue from human pluripotent stem cell-derived spheroids. Stem Cell Rev Rep. 12:500–508. DOI: 10.1007/s12015-016-9657-5. PMID: 27138846. PMCID: PMC6186008.
Article23. Ober EA, Lemaigre FP. 2018; Development of the liver: insights into organ and tissue morphogenesis. J Hepatol. 68:1049–1062. DOI: 10.1016/j.jhep.2018.01.005. PMID: 29339113.
Article24. Trépo E, Romeo S, Zucman-Rossi J, Nahon P. 2016; PNPLA3 gene in liver diseases. J Hepatol. 65:399–412. DOI: 10.1016/j.jhep.2016.03.011. PMID: 27038645.
Article25. Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM, Siveke JT. 2008; Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 48:607–616. DOI: 10.1002/hep.22381. PMID: 18666240.
Article26. Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang KJ, Karalis K, Kim HJ, MacQueen L, Mahmoodian R, Musah S, Torisawa YS, van der Meer AD, Villenave R, Yadid M, Parker KK, Ingber DE. 2015; Engineered in vitro disease models. Annu Rev Pathol. 10:195–262. DOI: 10.1146/annurev-pathol-012414-040418. PMID: 25621660.
Article27. Greek R, Menache A. 2013; Systematic reviews of animal models: methodology versus epistemology. Int J Med Sci. 10:206–221. DOI: 10.7150/ijms.5529. PMID: 23372426. PMCID: PMC3558708.
Article28. Yang L, Yang JL, Byrne S, Pan J, Church GM. 2014; CRISPR/Cas9-directed genome editing of cultured cells. Curr Protoc Mol Biol. 107:31.1.1–31.1.17. DOI: 10.1002/0471142727.mb3101s107. PMID: 24984853.
Article29. Guan Y, Xu D, Garfin PM, Ehmer U, Hurwitz M, Enns G, Michie S, Wu M, Zheng M, Nishimura T, Sage J, Peltz G. 2017; Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2:e94954. DOI: 10.1172/jci.insight.94954. PMID: 28878125. PMCID: PMC5621886.
Article30. Levy G, Bomze D, Heinz S, Ramachandran SD, Noerenberg A, Cohen M, Shibolet O, Sklan E, Braspenning J, Nahmias Y. 2015; Long-term culture and expansion of primary human hepatocytes. Nat Biotechnol. 33:1264–1271. DOI: 10.1038/nbt.3377. PMID: 26501953.
Article31. Avior Y, Levy G, Zimerman M, Kitsberg D, Schwartz R, Sadeh R, Moussaieff A, Cohen M, Itskovitz-Eldor J, Nahmias Y. 2015; Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology. 62:265–278. DOI: 10.1002/hep.27803. PMID: 25808545.
Article32. Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, Lee HJ, Kim J, Jung CR, Chung KS, Son MJ. 2019; Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 71:970–985. DOI: 10.1016/j.jhep.2019.06.030. PMID: 31299272.
Article33. Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz- Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. 2008; Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 26:313–315. DOI: 10.1038/nbt1383. PMID: 18278034.
Article34. Närvä E, Autio R, Rahkonen N, Kong L, Harrison N, Kitsberg D, Borghese L, Itskovitz-Eldor J, Rasool O, Dvorak P, Hovatta O, Otonkoski T, Tuuri T, Cui W, Brüstle O, Baker D, Maltby E, Moore HD, Benvenisty N, Andrews PW, Yli-Harja O, Lahesmaa R. 2010; High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozy-gosity. Nat Biotechnol. 28:371–377. DOI: 10.1038/nbt.1615. PMID: 20351689.
Article35. Chen XM, Kan QC, Wang F, Kong HJ, Zhang YY, Yu WZ, Sun YP. 2012; Chromosome dynamic changes in two cultured Chinese human embryonic stem cell lines: single nucleotide polymorphism, copy number variation and loss of heterozygosity. J Cell Biochem. 113:3520–3527. DOI: 10.1002/jcb.24229. PMID: 22711576.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vascularized Organoids: A More Complete Model
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine
- Human Pluripotent Stem Cell-Derived Retinal Organoids: A Viable Platform for Investigating the Efficacy of Adeno-Associated Virus Gene Therapy
- Region Specific Brain Organoids to Study Neurodevelopmental Disorders