Int J Stem Cells.
2021 Feb;14(1):112-118. 10.15283/ijsc20117.
Efficacy of Gene Modification in Placenta-Derived Mesenchymal Stem Cells Based on Nonviral Electroporation
- Affiliations
-
- 1Department of Biomedical Science, CHA University, Seongnam, Korea
- 2Department of Oral Pathology, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea
- 3Hamchoon Women’s clinic, Research Center of Fertility & Genetics, Seoul, Korea
- KMID: 2513088
- DOI: http://doi.org/10.15283/ijsc20117
Abstract
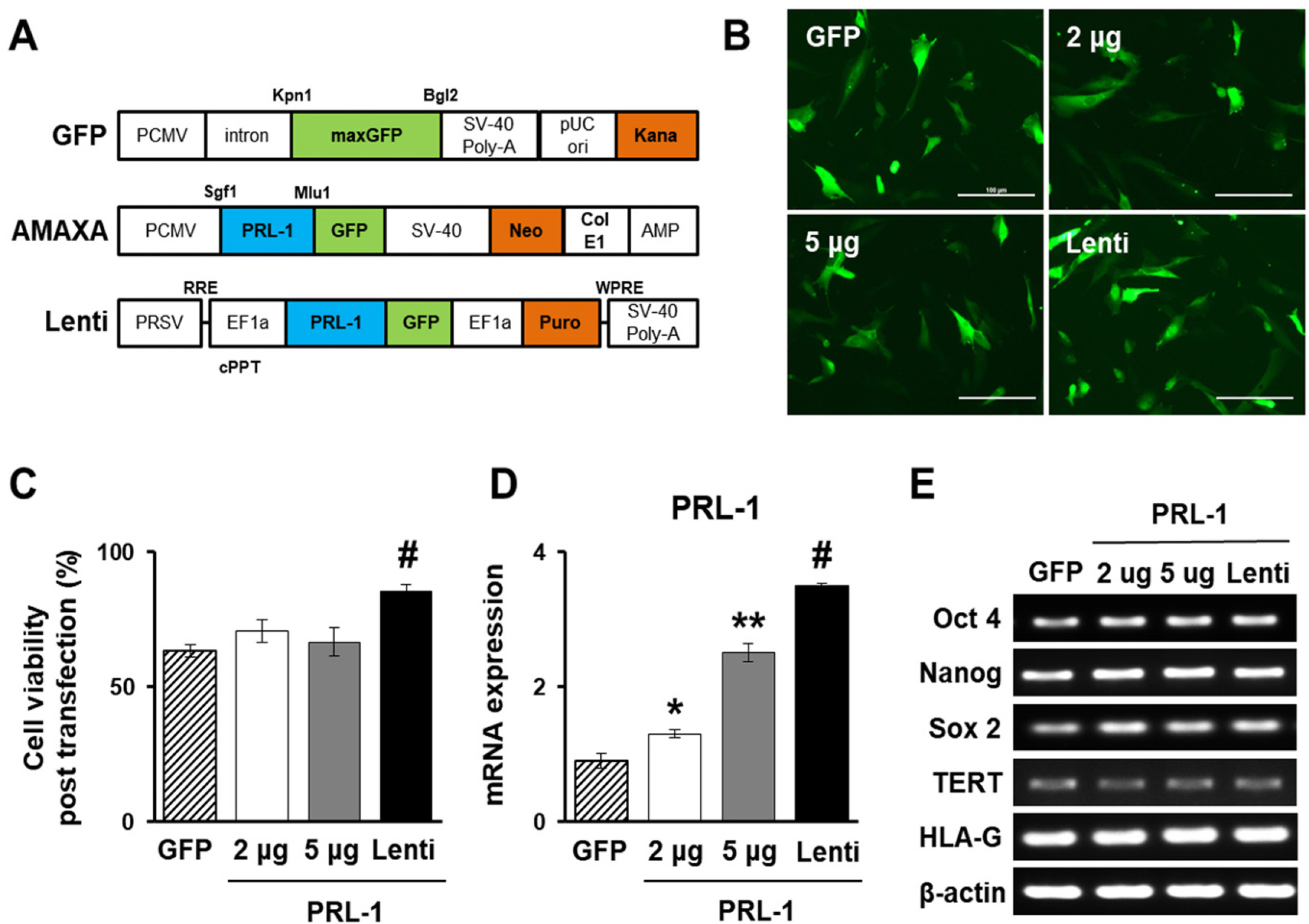
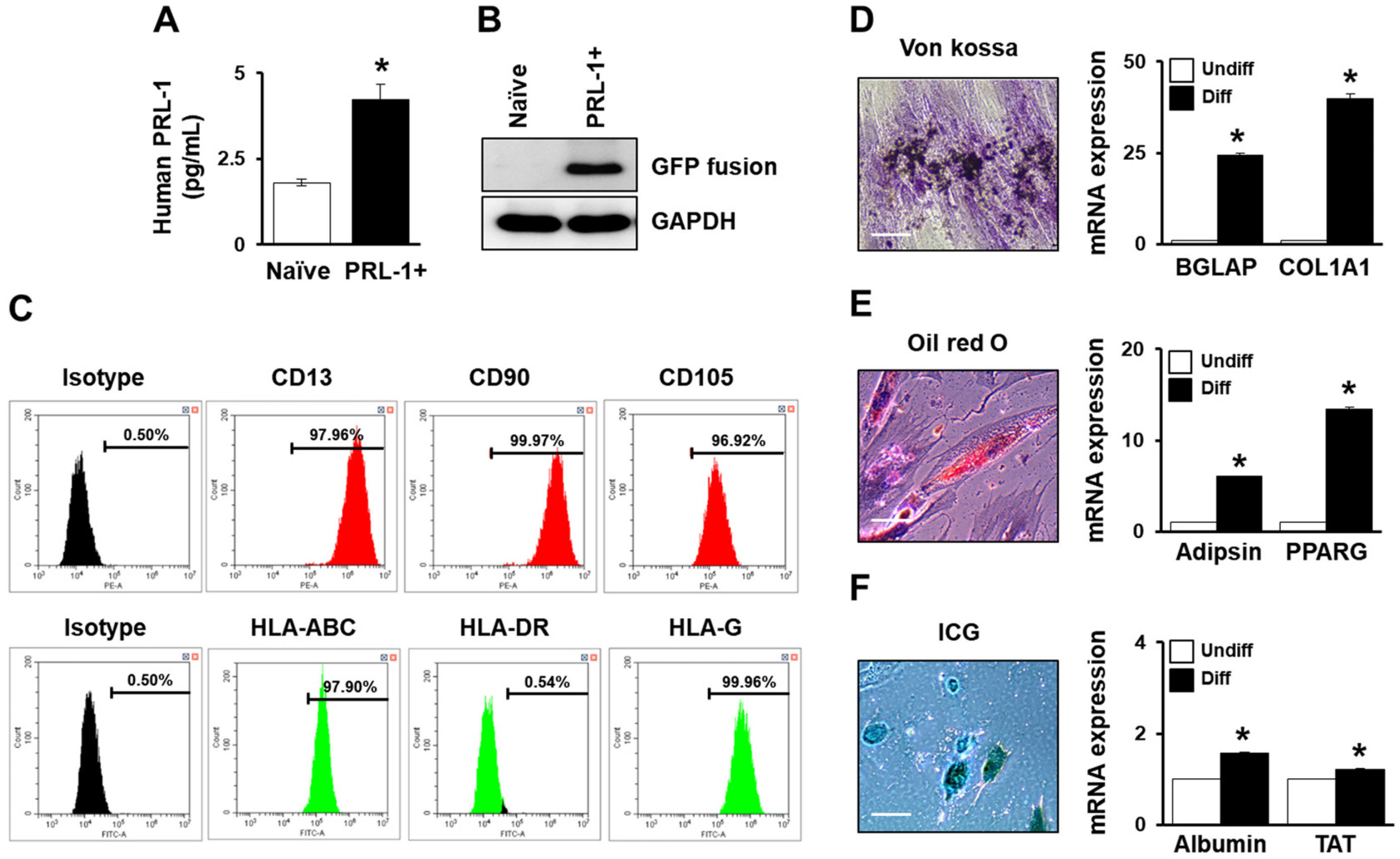
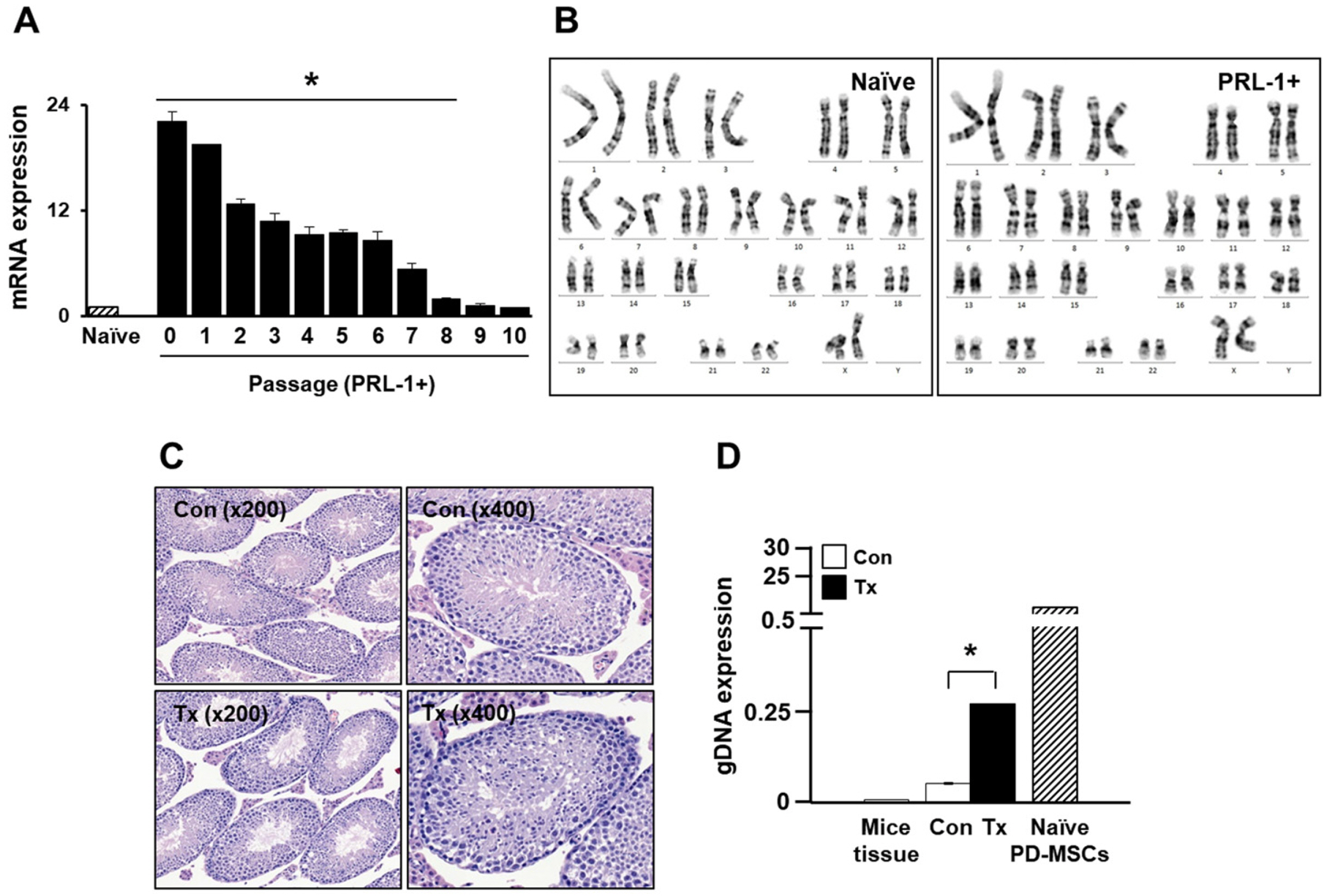
- Mesenchymal stem cell (MSC)-based therapy using gene delivery systems has been suggested for degenerative diseases. Although MSC-based clinical applications are effective and safe, the mode of action remains unclear. Researchers have commonly applied viral-based gene modification because this system has efficient vehicles. While viral transfection carries many risks, such as oncogenes and chromosomal integration, nonviral gene delivery techniques are less expensive, easier to handle, and safe, although they are less efficient. The electroporation method, which uses Nucleofection technology, provides critical opportunities for hard-to-transfect primary cell lines, including MSCs. Therefore, to improve the therapeutic efficacy using genetically modified MSCs, researchers must determine the optimal conditions for the introduction of the Nucleofection technique in MSCs. Here, we suggest optimal methods for gene modification in PD-MSCs using an electroporation gene delivery system for clinical application.
Keyword
Figure
Reference
-
References
1. Park JS, Suryaprakash S, Lao YH, Leong KW. 2015; Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods. 84:3–16. DOI: 10.1016/j.ymeth.2015.03.002. PMID: 25770356. PMCID: PMC4526354.
Article2. Ricks DM, Kutner R, Zhang XY, Welsh DA, Reiser J. 2008; Optimized lentiviral transduction of mouse bone marrow- derived mesenchymal stem cells. Stem Cells Dev. 17:441–450. DOI: 10.1089/scd.2007.0194. PMID: 18513160. PMCID: PMC2996877.
Article3. Ye Z, Lu W, Liang L, Tang M, Wang Y, Li Z, Zeng H, Wang A, Lin M, Huang L, Wang H, Hu H. 2019; Mesenchymal stem cells overexpressing hepatocyte nuclear factor-4 alpha alleviate liver injury by modulating anti-inflammatory functions in mice. Stem Cell Res Ther. 10:149. DOI: 10.1186/s13287-019-1260-7. PMID: 31133062. PMCID: PMC6537220.
Article4. Oggu GS, Sasikumar S, Reddy N, Ella KKR, Rao CM, Bokara KK. 2017; Gene delivery approaches for mesenchymal stem cell therapy: strategies to increase efficiency and specificity. Stem Cell Rev Rep. 13:725–740. DOI: 10.1007/s12015-017-9760-2. PMID: 28815481.
Article5. Santos JL, Pandita D, Rodrigues J, Pêgo AP, Granja PL, Tomás H. 2011; Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Curr Gene Ther. 11:46–57. DOI: 10.2174/156652311794520102. PMID: 21182464.
Article6. Lee HJ, Choi JH, Jung J, Kim JK, Lee SS, Kim GJ. 2014; Changes in PTTG1 by human TERT gene expression modulate the self-renewal of placenta-derived mesenchymal stem cells. Cell Tissue Res. 357:145–157. DOI: 10.1007/s00441-014-1874-0. PMID: 24816985.
Article7. Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, Cha DH, Yoon TK, Kim GJ. 2011; Human chorionic-plate-de-rived mesenchymal stem cells and Wharton's jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 346:53–64. DOI: 10.1007/s00441-011-1249-8. PMID: 21987220.
Article8. Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH, Kim GI, Kwon SW, Hwang SG, Kim GJ. 2010; Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: potential application to the treatment of hepatic diseases. J Cell Biochem. 111:1453–1463. DOI: 10.1002/jcb.22873. PMID: 20830742.
Article9. Lee HJ, Cha KE, Hwang SG, Kim JK, Kim GJ. 2011; In vitro screening system for hepatotoxicity: comparison of bone- marrow-derived mesenchymal stem cells and Placenta-derived stem cells. J Cell Biochem. 112:49–58. DOI: 10.1002/jcb.22728. PMID: 20524208.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Expression of embryonic stem cell markers in human term placenta
- Mesenchymal Stem Cell Therapy in Pulmonary Disease
- Comparison of Various Transfection Methods in Human and Bovine Cultured Cells
- Immunomodulatory Effects of Placenta-derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression