Int J Stem Cells.
2021 Feb;14(1):21-32. 10.15283/ijsc20088.
Autologous Bone-Marrow vs.Peripheral Blood Mononuclear Cells Therapy for Peripheral Artery Disease in Diabetic Patients
- Affiliations
-
- 1Department of Internal Medicine, Dr. Cipto Mangunkusumo National General Hospital, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia
- 2Metabolic Disorder, Cardiovascular, and Aging Cluster, Indonesian Medical Education and Research Institute, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia
- 3Department of Radiology, Dr. Cipto Mangunkusumo National General Hospital, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia
- KMID: 2513080
- DOI: http://doi.org/10.15283/ijsc20088
Abstract
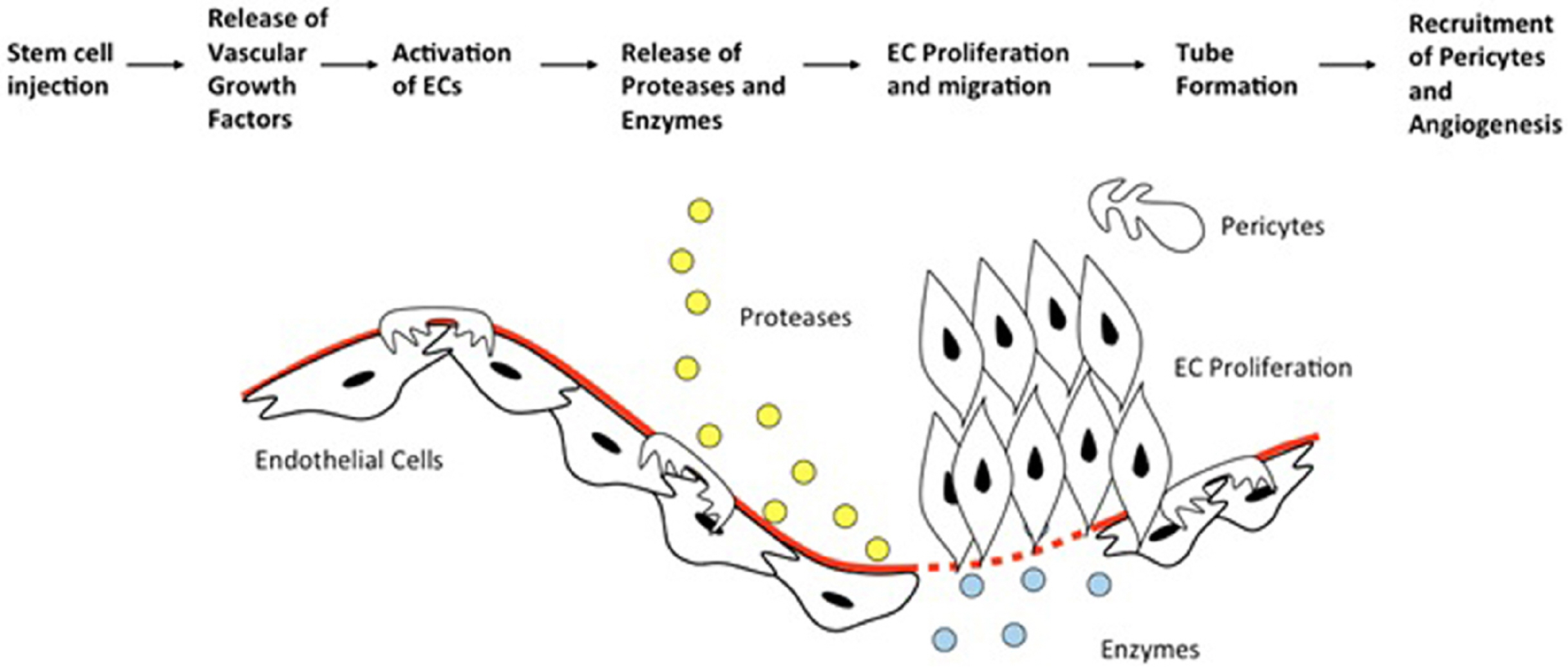
- Diabetes mellitus (DM) remains one of the most important risk factors for peripheral artery disease (PAD), with approximately 20% of DM patients older than 40 years old are affected with PAD. The current standard management for severe PAD is endovascular intervention with or without surgical bypass. Unfortunately, up to 40% of patients are unable to undergo these revascularization therapies due to excessive surgical risk or adverse vascular side effects. Stem cell therapy has emerged as a novel therapeutic strategy for these ‘no-option’ patients. Several types of stem cells are utilized for PAD therapy, including bone marrow mononuclear cells (BMMNC) and peripheral blood mononuclear cells (PBMNC). Many studies have reported the safety of BMMNC and PBMNC, as well as its efficacy in reducing ischemic pain, ulcer size, pain-free walking distance, ankle-brachial index (ABI), and transcutaneous oxygen pressure (TcPO2). However, the capacity to establish the efficacy of reducing major amputation rates, amputation free survival, and all-cause mortality is limited, as shown by several randomized placebo-controlled trials. The present literature review will focus on comparing safety and efficacy between BMMNC and PBMNC as cell-based management in diabetic patients with PAD who are not suitable for revascularization therapy.
Keyword
Figure
Reference
-
References
1. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. 2006; ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 47:1239–1312. DOI: 10.1016/j.jacc.2005.10.009. PMID: 16545667.2. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. 2013; Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 382:1329–1340. DOI: 10.1016/S0140-6736(13)61249-0. PMID: 23915883.
Article3. Marso SP, Hiatt WR. 2006; Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 47:921–929. DOI: 10.1016/j.jacc.2005.09.065. PMID: 16516072.
Article4. Kamil S, Sehested TSG, Carlson N, Houlind K, Lassen JF, N Bang C, Dominguez H, Pedersen CT, Gislason GH. 2019; Diabetes and risk of peripheral artery disease in patients undergoing first-time coronary angiography between 2000 and 2012- a nationwide study. BMC Cardiovasc Disord. 19:234. DOI: 10.1186/s12872-019-1213-1. PMID: 31651241. PMCID: PMC6813965.5. Maulahela H. 2013. Clinical outcome of percutaneus transluminal angioplasty in type 2 diabetic patient with peripheral arterial disease and distribution of factors that influenced clinical success [thesis]. Universitas Indonesia;Depok:
Article6. Thiruvoipati T, Kielhorn CE, Armstrong EJ. 2015; Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 6:961–969. DOI: 10.4239/wjd.v6.i7.961. PMID: 26185603. PMCID: PMC4499529.
Article7. Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. 2014; Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 60:686–695. e. DOI: 10.1016/j.jvs.2014.03.290. PMID: 24820900.
Article8. Sigvant B, Lundin F, Wahlberg E. 2016; The risk of disease progression in peripheral arterial disease is higher than expected: a meta-analysis of mortality and disease progression in peripheral arterial disease. Eur J Vasc Endovasc Surg. 51:395–403. DOI: 10.1016/j.ejvs.2015.10.022. PMID: 26777541.
Article9. Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. 2019; The burden of critical limb ischemia: a review of recent literature. Vasc Health Risk Manag. 15:187–208. DOI: 10.2147/VHRM.S209241. PMID: 31308682. PMCID: PMC6617560.10. Mills JL. 2016; Lower limb ischaemia in patients with diabetic foot ulcers and gangrene: recognition, anatomic patterns and revascularization strategies. Diabetes Metab Res Rev. 32 Suppl 1:239–245. DOI: 10.1002/dmrr.2753. PMID: 26455728.
Article11. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2017; 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American college of cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 135:e686–e725. DOI: 10.1161/CIR.0000000000000470. PMCID: PMC5479414. PMID: 27840332.
Article12. Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H. 2005; Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 366:1925–1934. DOI: 10.1016/S0140-6736(05)67704-5. PMID: 16325694.
Article13. Kalra K, Tomar PC. 2014; Stem cell: basics, classification and applications. Am J Phytomed Clin Ther. 2:919–930.14. Barky AR, Ali EMM, Mohamed TM. 2017; Stem cells, classifications and their clinical applications. Am J Pharmacol Ther. 1:1–7.15. Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. 2018; Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 15:36–45. DOI: 10.7150/ijms.21666. PMID: 29333086. PMCID: PMC5765738.
Article16. Silvestre JS, Mallat Z, Tedgui A, Lévy BI. 2008; Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 78:242–249. DOI: 10.1093/cvr/cvn027. PMID: 18252762.
Article17. Tuomisto TT, Rissanen TT, Vajanto I, Korkeela A, Rutanen J, Ylä-Herttuala S. 2004; HIF-VEGF-VEGFR-2, TNF- alpha and IGF pathways are upregulated in critical human skeletal muscle ischemia as studied with DNA array. Atherosclerosis. 174:111–120. DOI: 10.1016/j.atherosclerosis.2004.01.015. PMID: 15135259.
Article18. Urbich C, Dimmeler S. 2004; Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 95:343–353. DOI: 10.1161/01.RES.0000137877.89448.78. PMID: 15321944.19. Kolluru GK, Bir SC, Kevil CG. 2012; Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012:918267. DOI: 10.1155/2012/918267. PMID: 22611498. PMCID: PMC3348526.
Article20. Mendoza MG, Robles HV, Romo E, Rios A, Escalante B. 2007; Nitric oxide-dependent neovascularization role in the lower extremity disease. Curr Pharm Des. 13:3591–3596. DOI: 10.2174/138161207782794103. PMID: 18220796.
Article21. Santarelli JG, Udani V, Yung YC, Cheshier S, Wagers A, Brekken RA, Weissman I, Tse V. 2006; Incorporation of bone marrow-derived Flk-1-expressing CD34+ cells in the endothelium of tumor vessels in the mouse brain. Neuro-surgery. 59:374–382. discussion 374–382. DOI: 10.1227/01.NEU.0000222658.66878.CC. PMID: 16883178.
Article22. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. 1997; Isolation of putative progenitor endothelial cells for angio-genesis. Science. 275:964–967. DOI: 10.1126/science.275.5302.964. PMID: 9020076.
Article23. Gnecchi M, Zhang Z, Ni A, Dzau VJ. 2008; Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 103:1204–1219. DOI: 10.1161/CIRCRESAHA.108.176826. PMID: 19028920. PMCID: PMC2667788.
Article24. Xie J, March KL, Murphy MP. Mohler ER, Annex BH, editors. 2016. Bone marrow- derived cells: From the laboratory to the clinic. Regenerative Medicine for Peripheral Artery Disease. Elsevier Inc.;San Diego: p. 27–42. DOI: 10.1016/B978-0-12-801344-1.00003-6. PMCID: PMC4795089.25. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. 2003; Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 348:593–600. DOI: 10.1056/NEJMoa022287. PMID: 12584367.
Article26. Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G. 2004; Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 103:2577–2584. DOI: 10.1182/blood-2003-08-2770. PMID: 14630797.
Article27. Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S. 2005; CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 97:314–322. DOI: 10.1161/01.RES.0000177670.72216.9b. PMID: 16020753.
Article28. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. 2004; Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 104:2752–2760. DOI: 10.1182/blood-2004-04-1396. PMID: 15226175.
Article29. Fadini GP, Losordo D, Dimmeler S. 2012; Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 110:624–637. DOI: 10.1161/CIRCRESAHA.111.243386. PMID: 22343557. PMCID: PMC3382070.
Article30. Yoder MC. 2013; Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl). 91:285–295. DOI: 10.1007/s00109-013-1002-8. PMID: 23371317. PMCID: PMC3704045.
Article31. Thirumala S, Goebel WS, Woods EJ. 2013; Manufacturing and banking of mesenchymal stem cells. Expert Opin Biol Ther. 13:673–691. DOI: 10.1517/14712598.2013.763925. PMID: 23339745.
Article32. Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. 2005; Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 25:2542–2547. DOI: 10.1161/01.ATV.0000190701.92007.6d. PMID: 16224047.
Article33. Iwashima S, Ozaki T, Maruyama S, Saka Y, Kobori M, Omae K, Yamaguchi H, Niimi T, Toriyama K, Kamei Y, Torii S, Murohara T, Yuzawa Y, Kitagawa Y, Matsuo S. 2009; Novel culture system of mesenchymal stromal cells from human subcutaneous adipose tissue. Stem Cells Dev. 18:533–543. DOI: 10.1089/scd.2008.0358. PMID: 19055360.
Article34. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000; Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 97:13625–13630. DOI: 10.1073/pnas.240309797. PMID: 11087820. PMCID: PMC17626.35. Tomokiyo A, Yoshida S, Hamano S, Hasegawa D, Sugii H, Maeda H. 2018; Detection, characterization, and clinical application of mesenchymal stem cells in periodontal ligament tissue. Stem Cells Int. 2018:5450768. DOI: 10.1155/2018/5450768. PMID: 30224921. PMCID: PMC6129323.
Article36. Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. 2007; Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 24:508–520. DOI: 10.1089/neu.2006.0161. PMID: 17402856. PMCID: PMC2605398.
Article37. Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS. 2006; Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 24:1620–1626. DOI: 10.1634/stemcells.2005-0365. PMID: 16497946.
Article38. Romanov YA, Svintsitskaya VA, Smirnov VN. 2003; Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 21:105–110. DOI: 10.1634/stemcells.21-1-105. PMID: 12529557.
Article39. Xu Y, Meng H, Li C, Hao M, Wang Y, Yu Z, Li Q, Han J, Zhai Q, Qiu L. 2010; Umbilical cord-derived mesenchymal stem cells isolated by a novel explantation technique can differentiate into functional endothelial cells and promote revascularization. Stem Cells Dev. 19:1511–1522. DOI: 10.1089/scd.2009.0321. PMID: 20170363.
Article40. Oliveira MS, Barreto-Filho JB. 2015; Placental-derived stem cells: culture, differentiation and challenges. World J Stem Cells. 7:769–775. DOI: 10.4252/wjsc.v7.i4.769. PMID: 26029347. PMCID: PMC4444616.
Article41. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Horwitz E. Prockop Dj. 2006; Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article42. Petrini M, Pacini S, Trombi L, Fazzi R, Montali M, Ikehara S, Abraham NG. 2009; Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev. 18:857–866. DOI: 10.1089/scd.2008.0291. PMID: 18991503. PMCID: PMC3085824.
Article43. D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. 2004; Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 117(Pt 14):2971–2981. DOI: 10.1242/jcs.01103. PMID: 15173316.44. Subrammaniyan R, Amalorpavanathan J, Shankar R, Rajkumar M, Baskar S, Manjunath SR, Senthilkumar R, Murugan P, Srinivasan VR, Abraham S. 2011; Application of autologous bone marrow mononuclear cells in six patients with advanced chronic critical limb ischemia as a result of diabetes: our experience. Cytotherapy. 13:993–999. DOI: 10.3109/14653249.2011.579961. PMID: 21671823.
Article45. Ponemone V, Gupta S, Sethi D, Suthar M, Sharma M, Powell RJ, Harris KL, Jungla N, Arambam P, Kaul U, Seth A, Bukhari S. 2017; Safety and effectiveness of bone marrow cell concentrate in the treatment of chronic critical limb ischemia utilizing a rapid point-of-care system. Stem Cells Int. 2017:4137626. DOI: 10.1155/2017/4137626. PMID: 28194186. PMCID: PMC5282442.
Article46. Baker PK, Rhodes EG, Duguid JK. 1992; Effective concentration of bone marrow mononuclear cells using density gradient separation within an automated cell separator. Transfus Sci. 13:353–356. DOI: 10.1016/0955-3886(92)90147-9. PMID: 10148131.
Article47. Aktas M, Radke TF, Strauer BE, Wernet P, Kogler G. 2008; Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cyto-therapy. 10:203–211. DOI: 10.1080/14653240701851324. PMID: 18368599.
Article48. Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, Kevy SV, Jacobson MS, Heeschen C. 2008; Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 16:1059–1069. DOI: 10.3727/000000007783472363. PMID: 18351022.
Article49. Autissier P, Soulas C, Burdo TH, Williams KC. 2010; Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytometry A. 77:410–419. DOI: 10.1002/cyto.a.20859. PMID: 20099249.
Article50. Bogoslovsky T, Wang D, Maric D, Scattergood-Keepper L, Spatz M, Auh S, Hallenbeck J. 2013; Cryopreservation and enumeration of human endothelial progenitor and endothelial cells for clinical trials. J Blood Disord Transfus. 4:158. DOI: 10.4172/2155-9864.1000158. PMID: 25309814. PMCID: PMC4193669.
Article51. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. 1999; Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 5:434–438. DOI: 10.1038/7434. PMID: 10202935.
Article52. Shepherd RM, Capoccia BJ, Devine SM, Dipersio J, Trinkaus KM, Ingram D, Link DC. 2006; Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 108:3662–3667. DOI: 10.1182/blood-2006-06-030577. PMID: 16912220. PMCID: PMC1895468.
Article53. Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, Martin H, Haendeler J, Zeiher AM, Dimmeler S. 2006; Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 26:2238–2243. DOI: 10.1161/01.ATV.0000240248.55172.dd. PMID: 16902165.
Article54. Bhattacharya P, Thiruppathi M, Elshabrawy HA, Alharshawi K, Kumar P, Prabhakar BS. 2015; GM-CSF: an immune modulatory cytokine that can suppress autoimmunity. Cytokine. 75:261–271. DOI: 10.1016/j.cyto.2015.05.030. PMID: 26113402. PMCID: PMC4553090.
Article55. Gazitt Y. 2000; Immunologic profiles of effector cells and peripheral blood stem cells mobilized with different hematopoietic growth factors. Stem Cells. 18:390–398. DOI: 10.1634/stemcells.18-6-390. PMID: 11072026.
Article56. Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. 2005; Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 28:2155–2160. DOI: 10.2337/diacare.28.9.2155. PMID: 16123483.
Article57. Ozturk A, Kucukardali Y, Tangi F, Erikci A, Uzun G, Bashekim C, Sen H, Terekeci H, Narin Y, Ozyurt M, Ozkan S, Sayan O, Rodop O, Nalbant S, Sıldıroglu O, Yalnız FF, Senkal IV, Sabuncu H, Oktenli C. 2012; Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J Diabetes Complications. 26:29–33. DOI: 10.1016/j.jdiacomp.2011.11.007. PMID: 22240264.
Article58. Minamino K, Adachi Y, Okigaki M, Ito H, Togawa Y, Fujita K, Tomita M, Suzuki Y, Zhang Y, Iwasaki M, Nakano K, Koike Y, Matsubara H, Iwasaka T, Matsumura M, Ikehara S. 2005; Macrophage colony-stimulating factor (M- CSF), as well as granulocyte colony-stimulating factor (G- CSF), accelerates neovascularization. Stem Cells. 23:347–354. DOI: 10.1634/stemcells.2004-0190. PMID: 15749929.
Article59. Lane TA, Law P, Maruyama M, Young D, Burgess J, Mullen M, Mealiffe M, Terstappen LW, Hardwick A, Moubayed M, Oldham F, Corringham RET, Ho AD. 1995; Harvesting and enrichment of hematopoietic progenitor cells mobilized into the peripheral blood of normal donors by granulocyte-mac-rophage colony-stimulating factor (GM-CSF) or G-CSF: potential role in allogeneic marrow transplantation. Blood. 85:275–282. DOI: 10.1182/blood.V85.1.275.bloodjournal851275. PMID: 7528570.
Article60. Fischmeister G, Kurz M, Haas OA, Micksche M, Buchinger P, Printz D, Ressmann G, Stroebel T, Peters C, Fritsch G, Gadner H. 1999; G-CSF versus GM-CSF for stimulation of peripheral blood progenitor cells (PBPC) and leukocytes in healthy volunteers: comparison of efficacy and tolerability. Ann Hematol. 78:117–123. DOI: 10.1007/s002770050487. PMID: 10211753.
Article61. Duong HK, Savani BN, Copelan E, Devine S, Costa LJ, Wingard JR, Shaughnessy P, Majhail N, Perales MA, Cutler CS, Bensinger W, Litzow MR, Mohty M, Champlin RE, Leather H, Giralt S, Carpenter PA. 2014; Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 20:1262–1273. DOI: 10.1016/j.bbmt.2014.05.003. PMID: 24816581.
Article62. Hsu JW, Wingard JR, Logan BR, Chitphakdithai P, Akpek G, Anderlini P, Artz AS, Bredeson C, Goldstein S, Hale G, Hematti P, Joshi S, Kamble RT, Lazarus HM, O'Donnell PV, Pulsipher MA, Savani BN, Schears RM, Shaw BE, Confer DL. 2015; Race and ethnicity influences collection of granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells from unrelated donors, a Center for International Blood and Marrow Transplant Research analysis. Biol Blood Marrow Transplant. 21:165–171. DOI: 10.1016/j.bbmt.2014.10.007. PMID: 25316111. PMCID: PMC4272878.
Article63. Poole J, Mavromatis K, Binongo JN, Khan A, Li Q, Khayata M, Rocco E, Topel M, Zhang X, Brown C, Corriere MA, Murrow J, Sher S, Clement S, Ashraf K, Rashed A, Kabbany T, Neuman R, Morris A, Ali A, Hayek S, Oshinski J, Yoon YS, Waller EK, Quyyumi AA. 2013; Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: a randomized clinical trial. JAMA. 310:2631–2639. DOI: 10.1001/jama.2013.282540. PMID: 24247554.
Article64. Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. 2007; Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 98:1335–1342. DOI: 10.1160/TH07-02-0137. PMID: 18064333.
Article65. Dubsky M, Jirkovska A, Bem R, Fejfarova V, Pagacova L, Sixta B, Varga M, Langkramer S, Sykova E, Jude EB. 2013; Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab Res Rev. 29:369–376. DOI: 10.1002/dmrr.2399. PMID: 23390092.
Article66. Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. 2002; Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke. 33:1362–1368. DOI: 10.1161/01.STR.0000014925.09415.C3. PMID: 11988616.
Article67. Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, Nickenig G. 2002; Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 22:1567–1572. DOI: 10.1161/01.ATV.0000036417.43987.D8. PMID: 12377731.
Article68. Reddy K, Zhou Z, Schadler K, Jia SF, Kleinerman ES. 2008; Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing's tumor vessels. Mol Cancer Res. 6:929–936. DOI: 10.1158/1541-7786.MCR-07-2189. PMID: 18567797. PMCID: PMC2441901.
Article69. Göthert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Göttgens B, Izon DJ, Begley CG. 2004; Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 104:1769–1777. DOI: 10.1182/blood-2003-11-3952. PMID: 15187022.
Article70. Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL, Salven P. 2008; Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 105:6620–6625. DOI: 10.1073/pnas.0710516105. PMID: 18443294. PMCID: PMC2365563.
Article71. Attwell D, Mishra A, Hall CN, O'Farrell FM, Dalkara T. 2016; What is a pericyte? J Cereb Blood Flow Metab. 36:451–455. DOI: 10.1177/0271678X15610340. PMID: 26661200. PMCID: PMC4759679.
Article72. Bergers G, Song S. 2005; The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 7:452–464. DOI: 10.1215/S1152851705000232. PMID: 16212810. PMCID: PMC1871727.
Article73. Caporarello N, D'Angeli F, Cambria MT, Candido S, Giallongo C, Salmeri M, Lombardo C, Longo A, Giurdanella G, Anfuso CD, Lupo G. 2019; Pericytes in microvessels: from "mural" function to brain and retina regeneration. Int J Mol Sci. 20:6351. DOI: 10.3390/ijms20246351. PMID: 31861092. PMCID: PMC6940987.
Article74. Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. 2004; Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 104:2084–2086. DOI: 10.1182/blood-2004-01-0336. PMID: 15191949. PMCID: PMC2698665.
Article75. Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. 2004; Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 94:230–238. DOI: 10.1161/01.RES.0000110419.50982.1C. PMID: 14656934.
Article76. Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. 2001; Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 104:1046–1052. DOI: 10.1161/hc3501.093817. PMID: 11524400.
Article77. Rehman J, Li J, Orschell CM, March KL. 2003; Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 107:1164–1169. DOI: 10.1161/01.CIR.0000058702.69484.A0. PMID: 12615796.
Article78. Dokun AO, Annex BH. 2011; The VEGF165b "ICE-o-form" puts a chill on the VEGF story. Circ Res. 109:246–247. DOI: 10.1161/CIRCRESAHA.111.249953. PMID: 21778432. PMCID: PMC3196354.
Article79. Carmeliet P, Jain RK. 2011; Molecular mechanisms and clinical applications of angiogenesis. Nature. 473:298–307. DOI: 10.1038/nature10144. PMID: 21593862. PMCID: PMC4049445.
Article80. Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. 1986; Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 83:7297–7301. DOI: 10.1073/pnas.83.19.7297. PMID: 2429303. PMCID: PMC386703.
Article81. Murakami M, Simons M. 2008; Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 15:215–220. DOI: 10.1097/MOH.0b013e3282f97d98. PMID: 18391788. PMCID: PMC2745288.
Article82. Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N, Gerritsen ME. 2001; Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 158:1111–1120. DOI: 10.1016/S0002-9440(10)64058-8. PMID: 11238059. PMCID: PMC1850376.
Article83. Sanada F, Taniyama Y, Azuma J, Yuka II, Kanbara Y, Iwabayashi M, Rakugi H, Morishita R. 2014; Therapeutic angiogenesis by gene therapy for critical limb ischemia: choice of biological agent. Immunol Endocr Metab Agents Med Chem. 14:32–39. DOI: 10.2174/1871522213999131231105139. PMID: 26005508. PMCID: PMC4435566.
Article84. Lawall H, Bramlage P, Amann B. 2010; Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 103:696–709. DOI: 10.1160/TH09-10-0688. PMID: 20174766.85. Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. 2002; Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 360:427–435. DOI: 10.1016/S0140-6736(02)09670-8. PMID: 12241713.
Article86. Persiani F, Paolini A, Camilli D, Mascellari L, Platone A, Magenta A, Furgiuele S. 2018; Peripheral blood mononuclear cells therapy for treatment of lower limb ischemia in diabetic patients: a single-center experience. Ann Vasc Surg. 53:190–196. DOI: 10.1016/j.avsg.2018.05.036. PMID: 30053546.
Article87. Horie T, Yamazaki S, Hanada S, Kobayashi S, Tsukamoto T, Haruna T, Sakaguchi K, Sakai K, Obara H, Morishita K, Saigo K, Shintani Y, Kubo K, Hoshino J, Oda T, Kaneko E, Nishikido M, Ioji T, Kaneda H, Fukushima M. 2018; Outcome from a randomized controlled clinical trial- improvement of peripheral arterial disease by granulocyte colony-stimulating factor-mobilized autologous peripheral-blood-mononuclear cell transplantation (IMPACT). Circ J. 82:2165–2174. DOI: 10.1253/circj.CJ-17-1220. PMID: 29877199.
Article88. Liew A, Bhattacharya V, Shaw J, Stansby G. 2016; Cell therapy for critical limb ischemia: a meta-analysis of randomized controlled trials. Angiology. 67:444–455. DOI: 10.1177/0003319715595172. PMID: 26195561.89. Wen Y, Meng L, Gao Q. 2011; Autologous bone marrow cell therapy for patients with peripheral arterial disease: a meta-analysis of randomized controlled trials. Expert Opin Biol Ther. 11:1581–1589. DOI: 10.1517/14712598.2011.626401. PMID: 21973046.
Article90. Ai M, Yan CF, Xia FC, Zhou SL, He J, Li CP. 2016; Safety and efficacy of cell-based therapy on critical limb ischemia: a meta-analysis. Cytotherapy. 18:712–724. DOI: 10.1016/j.jcyt.2016.02.009. PMID: 27067609.
Article91. Rigato M, Monami M, Fadini GP. 2017; Autologous cell therapy for peripheral arterial disease: systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 120:1326–1340. DOI: 10.1161/CIRCRESAHA.116.309045. PMID: 28096194.
Article92. Xie B, Luo H, Zhang Y, Wang Q, Zhou C, Xu D. 2018; Autologous stem cell therapy in critical limb ischemia: a meta-analysis of randomized controlled trials. Stem Cells Int. 2018:7528464. DOI: 10.1155/2018/7528464. PMID: 29977308. PMCID: PMC5994285.
Article93. Pan T, Wei Z, Fang Y, Dong Z, Fu W. 2018; Therapeutic efficacy of CD34+ cell-involved mononuclear cell therapy for no-option critical limb ischemia: a meta-analysis of randomized controlled clinical trials. Vasc Med. 23:219–231. DOI: 10.1177/1358863X17752556. PMID: 29457540.
Article94. Gao W, Chen D, Liu G, Ran X. 2019; Autologous stem cell therapy for peripheral arterial disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 10:140. DOI: 10.1186/s13287-019-1254-5. PMID: 31113463. PMCID: PMC6528204.
Article95. Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC, Moll FL. 2015; Bone marrow derived cell therapy in critical limb ischemia: a meta-analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg. 50:775–783. DOI: 10.1016/j.ejvs.2015.08.018. PMID: 26460286.
Article96. Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. 2006; Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 98:697–704. DOI: 10.1161/01.RES.0000209948.50943.ea. PMID: 16484619.
Article97. Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. 2006; Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 26:2140–2146. DOI: 10.1161/01.ATV.0000237750.44469.88. PMID: 16857948.
Article98. Tecilazich F, Dinh T, Pradhan-Nabzdyk L, Leal E, Tellechea A, Kafanas A, Gnardellis C, Magargee ML, Dejam A, Toxavidis V, Tigges JC, Carvalho E, Lyons TE, Veves A. 2013; Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One. 8:e83314. DOI: 10.1371/journal.pone.0083314. PMID: 24358275. PMCID: PMC3865213.
Article99. Zafar N, Krishnasamy SS, Shah J, Rai SN, Riggs DW, Bhatnagar A, O'Toole TE. 2018; Circulating angiogenic stem cells in type 2 diabetes are associated with glycemic control and endothelial dysfunction. PLoS One. 13:e0205851. DOI: 10.1371/journal.pone.0205851. PMID: 30321232. PMCID: PMC6188890.
Article100. Zhou B, Bi YY, Han ZB, Ren H, Fang ZH, Yu XF, Poon MC, Han ZC. 2006; G-CSF-mobilized peripheral blood mononuclear cells from diabetic patients augment neovascula-rization in ischemic limbs but with impaired capability. J Thromb Haemost. 4:993–1002. DOI: 10.1111/j.1538-7836.2006.01906.x. PMID: 16689750.
Article101. Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, Teodorescu V, Wiechmann BN, Thompson C, Kraiss L, Carman T, Dohad S, Huang P, Junge CE, Story K, Weistroffer T, Thorne TM, Millay M, Runyon JP, Schainfeld R. 2012; A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 5:821–830. DOI: 10.1161/CIRCINTERVENTIONS.112.968321. PMID: 23192920. PMCID: PMC3549397.
Article102. Klepanec A, Mistrik M, Altaner C, Valachovicova M, Olejarova I, Slysko R, Balazs T, Urlandova T, Hladikova D, Liska B, Tomka J, Vulev I, Madaric J. 2012; No difference in intra-arterial and intramuscular delivery of autologous bone marrow cells in patients with advanced critical limb ischemia. Cell Transplant. 21:1909–1918. DOI: 10.3727/096368912X636948. PMID: 22472173.
Article103. Gu YQ, Zhang J, Guo LR, Qi LX, Zhang SW, Xu J, Li JX, Luo T, Ji BX, Li XF, Yu HX, Cui SJ, Wang ZG. 2008; Transplantation of autologous bone marrow mononuclear cells for patients with lower limb ischemia. Chin Med J (Engl). 121:963–967. DOI: 10.1097/00029330-200806010-00001. PMID: 18706241.
Article104. Patiño B, Espinosa D, Solano MH, Abello V, Casas C. 2018; Morbidity and mortality associated with performing bone marrow aspiration and biopsy. Int Phys Med Rehab J. 3:65–70. DOI: 10.15406/ipmrj.2018.03.00077.
Article105. Hölig K. 2013; G-CSF in healthy allogeneic stem cell donors. Transfus Med Hemother. 40:225–235. DOI: 10.1159/000354196. PMID: 24179471. PMCID: PMC3776391.
Article106. Balaguer H, Galmes A, Ventayol G, Bargay J, Besalduch J. 2004; Splenic rupture after granulocyte-colony-stimulating factor mobilization in a peripheral blood progenitor cell donor. Transfusion. 44:1260–1261. DOI: 10.1111/j.1537-2995.2004.00413.x. PMID: 15265137.
Article107. Azoulay E, Attalah H, Harf A, Schlemmer B, Delclaux C. 2001; Granulocyte colony-stimulating factor or neutrophil-induced pulmonary toxicity: myth or reality? Systematic review of clinical case reports and experimental data. Chest. 120:1695–1701. DOI: 10.1378/chest.120.5.1695. PMID: 11713155.
Article108. Falanga A, Marchetti M, Evangelista V, Manarini S, Oldani E, Giovanelli S, Galbusera M, Cerletti C, Barbui T. 1999; Neutrophil activation and hemostatic changes in healthy donors receiving granulocyte colony-stimulating factor. Blood. 93:2506–2514. DOI: 10.1182/blood.V93.8.2506. PMID: 10194429.
Article109. Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H. 2008; Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 156:1010–1018. DOI: 10.1016/j.ahj.2008.06.025. PMID: 19061721.
Article110. Onodera R, Teramukai S, Tanaka S, Kojima S, Horie T, Matoba S, Murohara T, Matsubara H, Fukushima M. 2011; Bone marrow mononuclear cells versus G-CSF-mobilized peripheral blood mononuclear cells for treatment of lower limb ASO: pooled analysis for long-term prognosis. Bone Marrow Transplant. 46:278–284. DOI: 10.1038/bmt.2010.110. PMID: 20479708.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mixed Mononuclear Cell Culture of Cord Blood and Adult Peripheral Blood
- Effect of Autologous Peripheral Blood Mononuclear Cell Transplantation on Patients with Chronic Ischemic Limb
- The Evolving Role of Myeloablative Chemotherapy with Stem Cell Transplantation for the Treatment of Autoimmune Disease
- Automated bone marrow processing with the blood cell separator
- A Comparison of the Chemistry of Blood from Bone Marrow and Peripheral Blood