Clin Exp Otorhinolaryngol.
2021 Feb;14(1):61-68. 10.21053/ceo.2019.01662.
Estrogen Replacement Reduces Hearing Threshold Shifts and Cochlear Hair Cell Loss After Acoustic Overexposure in Ovariectomized Rats
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Dankook University Hospital, Cheonan, Korea
- 2Department of Otolaryngology-Head and Neck Surgery, Dankook University College of Medicine, Cheonan, Korea
- 3Beckman Laser Institute Korea, Dankook University College of Medicine, Cheonan, Korea
- KMID: 2512882
- DOI: http://doi.org/10.21053/ceo.2019.01662
Abstract
Objectives
. The relationship of estrogen (the primary female sex hormone) with hearing function has been studied in both humans and animals. However, whether estrogen levels affect hearing remains uncertain. Therefore, in this study, we investigated changes in the vulnerability of hearing to acoustic overexposure in ovariectomized female rats.
Methods
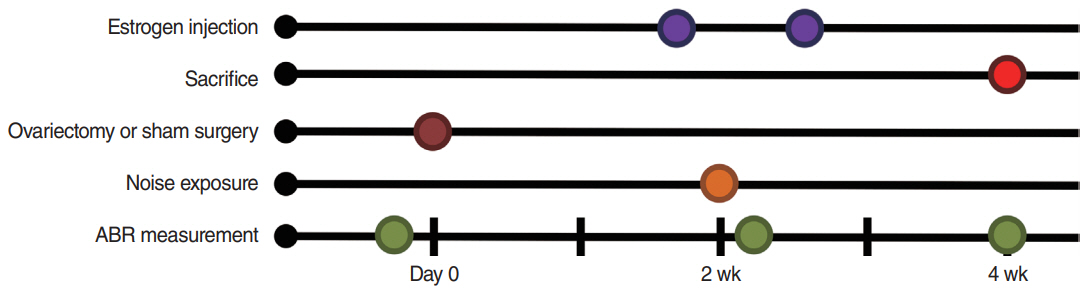
. Eighteen 8-week-old female Sprague-Dawley rats were separated into four groups as follows: sham ovariectomy (OP), OP only, and OP treated with low (10 µg/kg) or high doses (100 µg/kg) of estrogen. Rats in the estrogen replacement groups were given two intraperitoneal injections. Hearing thresholds were measured before noise exposure, and at 1 day and 2 weeks after exposure.
Results
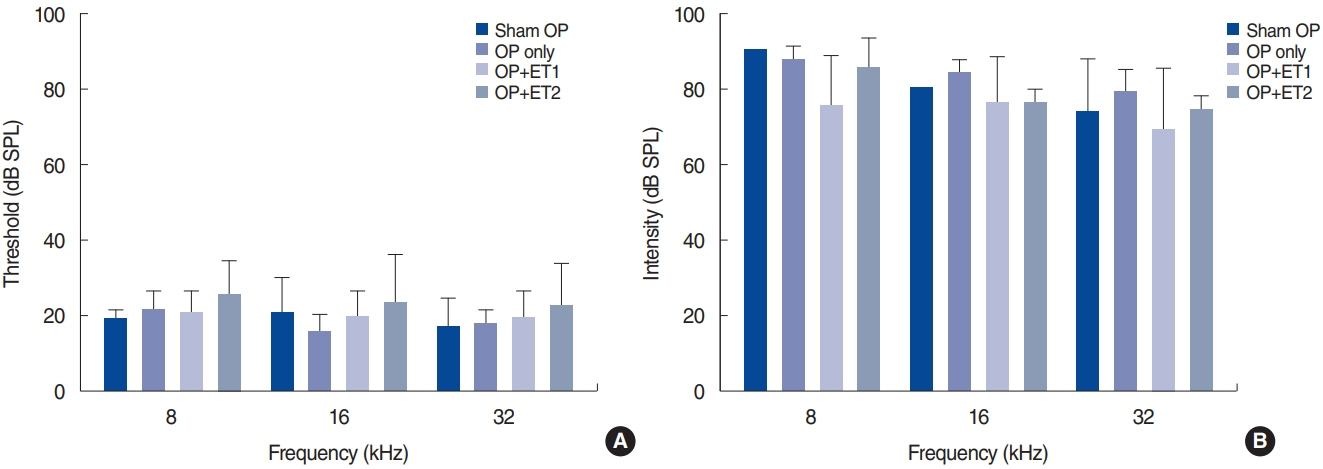
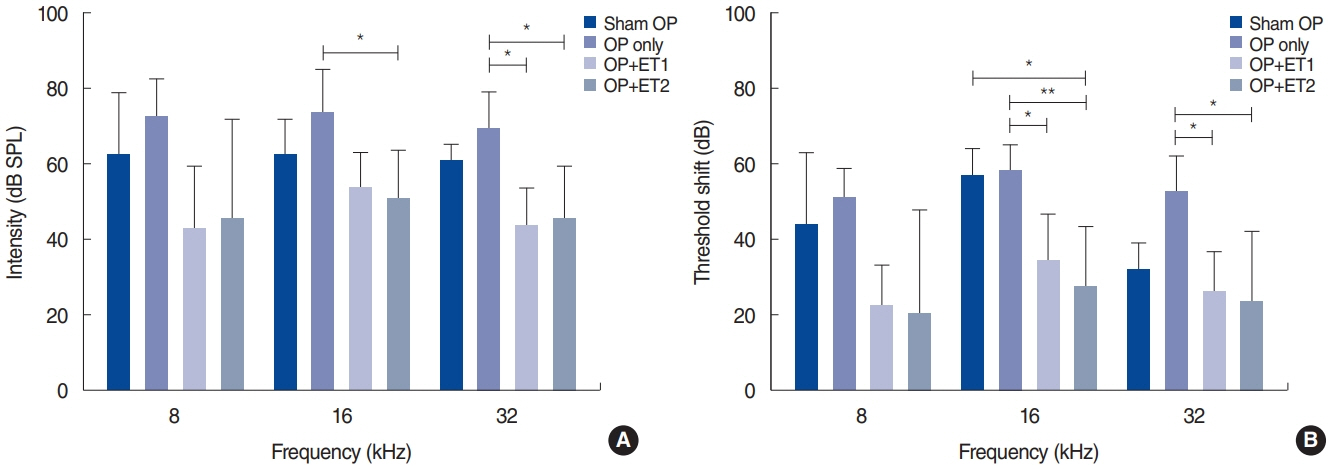
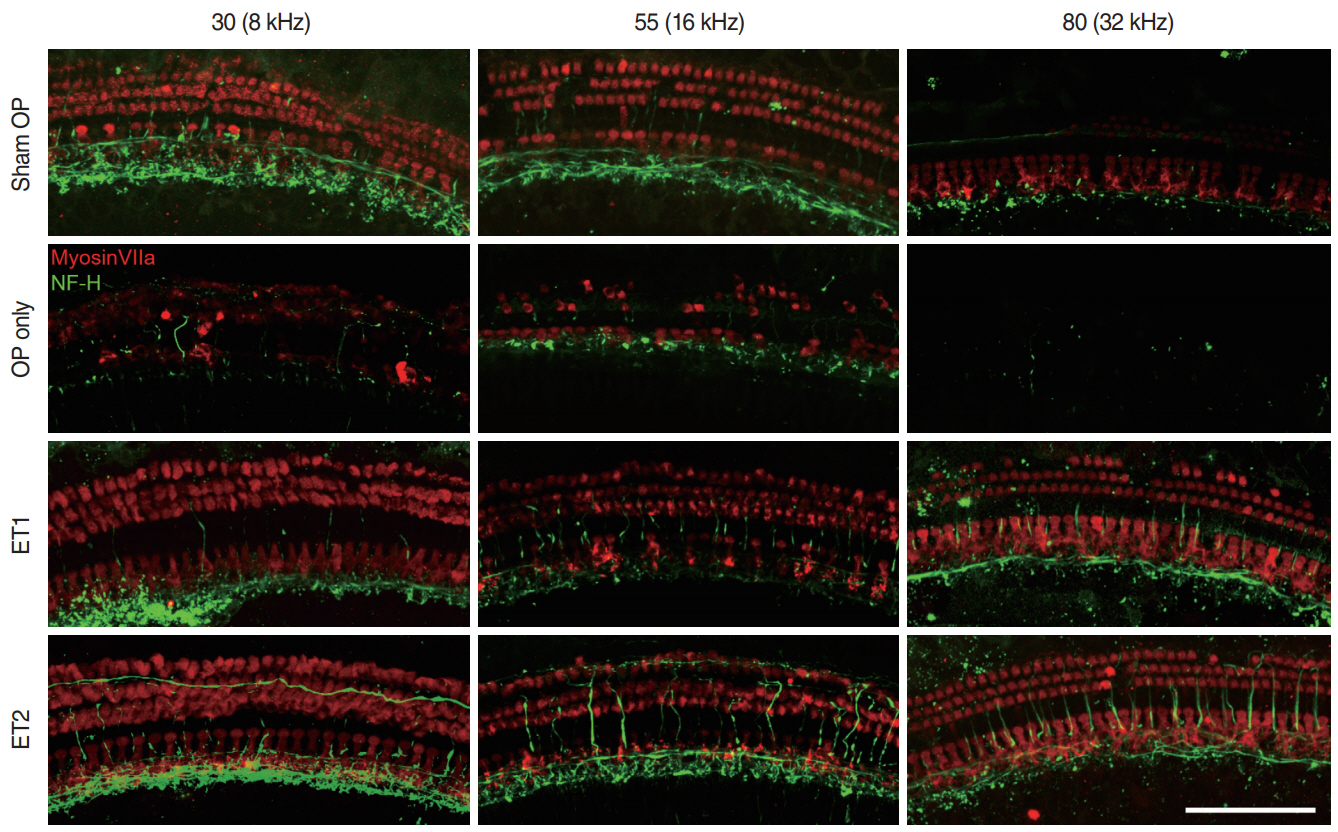
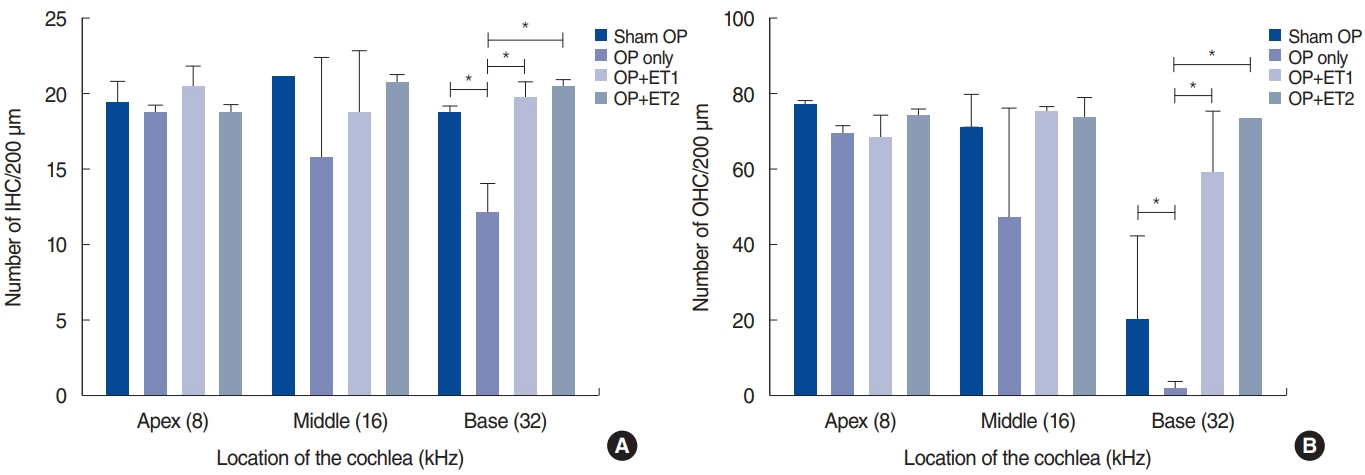
. The hearing thresholds of the sham OP and OP-only groups were not significantly different. However, both estrogen groups showed a lower threshold shift than the OP-only group. Histological immunostaining analyses showed that hair cell loss in the 32 kHz region was more severe in the sham OP group than in the OP-only group. Furthermore, there was little or no hair cell loss in either estrogen replacement group and significantly more hair cell loss in the OP-only group.
Conclusion
. These results suggest that estrogen replacement may reduce the vulnerability of hearing to noise exposure in menopausal women.
Figure
Cited by 1 articles
-
Do Women Have Better Hearing Than Men?
Yong-Ho Park
Clin Exp Otorhinolaryngol. 2021;14(1):1-2. doi: 10.21053/ceo.2020.02418.
Reference
-
1. Hamernik RP, Patterson JH, Turrentine GA, Ahroon WA. The quantitative relation between sensory cell loss and hearing thresholds. Hear Res. 1989; Apr. 38(3):199–211.
Article2. Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A, Sahlin L, et al. Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice. J Endocrinol. 2009; Jun. 201(3):397–406.3. Caruso S, Cianci A, Grasso D, Agnello C, Galvani F, Maiolino L, et al. Auditory brainstem response in postmenopausal women treated with hormone replacement therapy: a pilot study. Menopause. 2000; May-Jun. 7(3):178–83.
Article4. Stenberg AE, Wang H, Fish J 3rd, Schrott-Fischer A, Sahlin L, Hultcrantz M. Estrogen receptors in the normal adult and developing human inner ear and in Turner’s syndrome. Hear Res. 2001; Jul. 157(1-2):87–92.
Article5. Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002; Jan. 143(1):205–12.
Article6. Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A. 1999; Aug. 96(16):8867–72.
Article7. Nakamagoe M, Tabuchi K, Uemaetomari I, Nishimura B, Hara A. Estradiol protects the cochlea against gentamicin ototoxicity through inhibition of the JNK pathway. Hear Res. 2010; Mar. 261(1-2):67–74.
Article8. Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994; Nov. 80(2):209–15.
Article9. Bittar RS, Cruz OL, Lorenzi MC, Marone SA, Miniti A. Morphological and functional study of the cochlea after administration of estrogen and progesterone in the guinea pig. Int Tinnitus J. 2001; 7(1):41–5.10. Golub MS, Germann SL, Hogrefe CE. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicol Teratol. 2004; Nov-Dec. 26(6):799–809.
Article11. Willott JF, VandenBosche J, Shimizu T, Ding DL, Salvi R. Effects of exposing gonadectomized and intact C57BL/6J mice to a high-frequency augmented acoustic environment: auditory brainstem response thresholds and cytocochleograms. Hear Res. 2006; Nov. 221(1-2):73–81.
Article12. Kim JH, Cho HT, Kim YJ. The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr J. 2014; 61(11):1055–67.13. Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001; May. 155(1-2):1–8.
Article14. Hultcrantz M, Sylven L, Borg E. Ear and hearing problems in 44 middle-aged women with Turner’s syndrome. Hear Res. 1994; Jun. 76(1-2):127–32.
Article15. Wilcox SA, Saunders K, Osborn AH, Arnold A, Wunderlich J, Kelly T, et al. High frequency hearing loss correlated with mutations in the GJB2 gene. Hum Genet. 2000; Apr. 106(4):399–405.
Article16. Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, et al. Estrogen receptor beta protects against acoustic trauma in mice: version 2. J Clin Invest. 2008; Apr. 118(4):1563–70.17. Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003; Jan. 144(1):306–12.
Article18. Lee MY, Kabara LL, Swiderski DL, Raphael Y, Duncan RK, Kim YH. ROS scavenger, ebselen, has no preventive effect in new hearing loss model using a cholesterol-chelating agent. J Audiol Otol. 2019; Apr. 23(2):69–75.
Article19. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017; Oct. 8(1):33.
Article20. Wronski TJ, Cintron M, Doherty AL, Dann LM. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988; Aug. 123(2):681–6.
Article21. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss: version 2. J Neurosci. 2009; Nov. 29(45):14077–85.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Cochlear Nerve Terminals after Temporary Noise-Induced Hearing Loss
- A Case of Electric Acoustic Stimulation Cochlear Implantation in Partial Deafness with Residual Low-Frequency Hearing
- DPOAE in Acoustic Neuroma
- Effect of Quercetin on Recovery of Hearing Threshold in Noise-Induced HSP72 Model
- The Past and Present of the Research on Cochlear Stem Cell