Lab Med Online.
2020 Apr;10(2):132-136. 10.3343/lmo.2020.10.2.132.
Evaluation of Intact Parathyroid Hormone Levels in Plasma Samples: A Comparative Study Using Serum Samples
- Affiliations
-
- 1Department of Laboratory Medicine, Korea Cancer Center Hospital, Seoul, Korea
- KMID: 2512243
- DOI: http://doi.org/10.3343/lmo.2020.10.2.132
Abstract
- Background
Intraoperative measurement of intact parathyroid hormone (iPTH) levels is crucial for confirming complete removal of hyperfunctioning parathyroid glands during parathyroidectomy and for detecting parathyroid gland damage during thyroidectomy. The use of plasma samples can shorten the turnaround time (TAT) for iPTH. The present study explored the effectiveness of using plasma samples for iPTH quantitation by comparison with the corresponding serum samples. We also evaluated the analytical performance of iPTH.
Methods
The TAT of plasma and serum samples analyzed in March 2019 was compared. In addition, comparative evaluation of the iPTH levels in 100 paired plasma and serum samples were performed. Analytical performances including within-run and within-laboratory precision, and linearity were evaluated in plasma samples using the ADVIA Centaur iPTH assay (Siemens Healthineers, Germany). The reference range was verified with plasma samples collected from 20 healthy adults.
Results
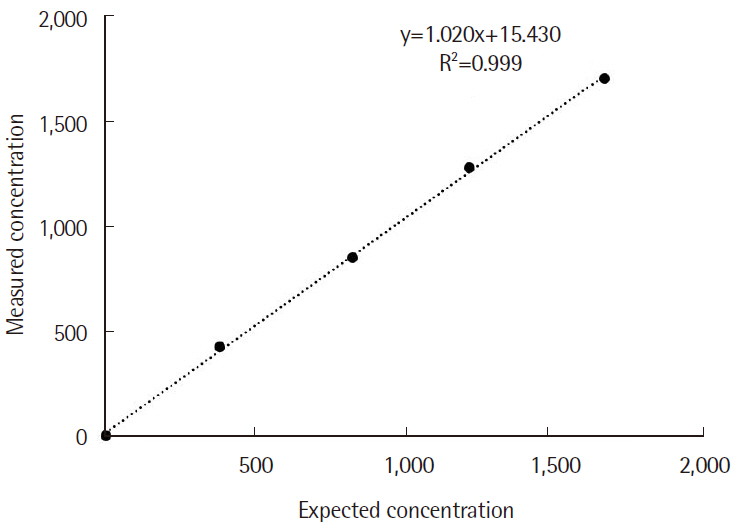
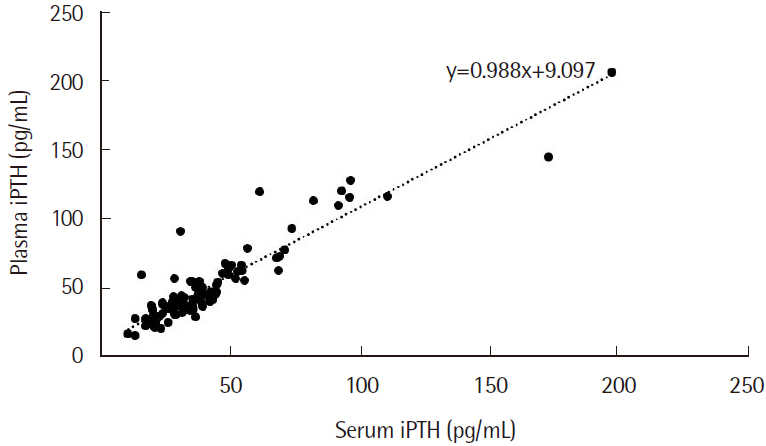
Plasma iPTH tests showed shorter TAT values (P<0.001) and higher iPTH levels (P<0.001) than serum. Correlation analysis between plasma and serum iPTH levels showed a strong positive correlation (r=0.925). The within-run and within-laboratory precision values were within the manufacturer's recommendation. iPTH showed linearity from 5.1 to 1,670.0 pg/mL (R2=0.999). The plasma iPTH levels from 20 healthy adults were within the reference range, thus validating our method.
Conclusions
The plasma iPTH levels were higher than the serum levels, with a strong positive correlation. The TAT of plasma samples was considerably shorter than that of serum. iPTH quantitation from plasma samples is preferable when rapid results are required.
Keyword
Figure
Reference
-
1. Mundy GR, Guise TA. 1999; Hormonal control of calcium homeostasis. Clin Chem. 45:1347–52. DOI: 10.1093/clinchem/45.8.1347. PMID: 10430817.
Article2. Friedman PA. 2000; Mechanisms of renal calcium transport. Exp Nephrol. 8:343–50. DOI: 10.1159/000020688. PMID: 11014931.
Article3. Goettsch C, Iwata H, Aikawa E. 2014; Parathyroid hormone: Critical bridge between bone metabolism and cardiovascular disease. Arterioscler Thromb Vasc Biol. 34:1333–5. DOI: 10.1161/ATVBAHA.114.303637. PMID: 24951650. PMCID: PMC4094346.
Article4. Bilezikian JP, Khan A, Potts JT Jr, Brandi ML, Clarke BL, Shoback D, et al. 2011; Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 26:2317–37. DOI: 10.1002/jbmr.483. PMID: 21812031. PMCID: PMC3405491.
Article5. Fraser WD. 2009; Hyperparathyroidism. Lancet. 374:145–58. DOI: 10.1016/S0140-6736(09)60507-9.
Article6. Adhikary LP, Yadava SK, Pokharel A, Khadka D, Thakur R. 2015; Relation between calcium, phosphorus, calcium-phosphorus index and iPTH in chronic kidney disease patients. J Nepal Health Res Counc. 13:50–3. PMID: 26411713.7. Barczyński M, Golkowski F, Nawrot I. 2015; The current status of intraoperative iPTH assay in surgery for primary hyperparathyroidism. Gland Surg. 4:36–43. DOI: 10.3978/j.issn.2227-684X.2015.01.01. PMID: 25713778. PMCID: PMC4321047.8. Naik AH, Wani MA, Wani KA, Laway BA, Malik AA, Shah ZA. 2018; Intraoperative parathyroid hormone monitoring in guiding adequate parathyroidectomy. Indian J Endocrinol Metab. 22:410–6. DOI: 10.4103/ijem.IJEM_678_17. PMID: 30090736. PMCID: PMC6063190.
Article9. Carr AA, Yen TW, Wilson SD, Evans DB, Wang TS. 2017; Using parathyroid hormone spikes during parathyroidectomy to guide intraoperative decision-making. J Surg Res. 209:162–7. DOI: 10.1016/j.jss.2016.10.006. PMID: 28032553.
Article10. Demeester-Mirkine N, Hooghe L, Van Geertruyden J, De Maertelaer V. 1992; Hypocalcemia after thyroidectomy. Arch Surg. 127:854–8. DOI: 10.1001/archsurg.1992.01420070118021. PMID: 1524486.
Article11. Lindblom P, Westerdahl J, Bergenfelz A. 2002; Low parathyroid hormone levels after thyroid surgery: a feasible predictor of hypocalcemia. Sur-gery. 131:515–20. DOI: 10.1067/msy.2002.123005. PMID: 12019404.
Article12. Lombardi CP, Raffaelli M, Princi P, Santini S, Boscherini M, De Crea C, et al. 2004; Early prediction of post thyroidectomy hypocalcemia by one single iPTH measurement. Surgery. 136:1236–41. DOI: 10.1016/j.surg.2004.06.053. PMID: 15657581.13. Lee DR, Hinson AM, Siegel ER, Steelman SC, Bodenner DL, Stack BC Jr. 2015; Comparison of intraoperative versus postoperative parathyroid hormone levels to predict hypocalcemia earlier after total thyroidectomy. Otolaryngol Head Neck Surg. 153:343–9. DOI: 10.1177/0194599815596341. PMID: 26209077.
Article14. Souberbielle JC, Brazier F, Piketty ML, Cormier C, Minisola S, Cavalier E. 2017; How the reference values for serum parathyroid hormone concentration are (or should be) established? J Endocrinol Invest. 40:241–56. DOI: 10.1007/s40618-016-0553-2. PMID: 27696297.
Article15. Clinical and Laboratory Standards Institute. 2014; User verification of precision and estimation of bias; Approved guideline-third edition. CLSI document EP15-A3. Clinical and Laboratory Standards Institute;Wayne, PA:16. Clinical and Laboratory Standards Institute. 2003; Evaluation of the linearity of quantitative measurement procedures: A statistical approach; Approved guideline. CLSI document EP06-A. Clinical and Laboratory Standards Institute;Wayne, PA:17. Clinical and Laboratory Standards Institute. 2008; Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guideline-third edition. CLSI document EP28-A3. Clinical and Laboratory Standards Institute;Wayne, PA:18. Cavalier E, Delanaye P, Hubert P, Krzesinski JM, Chapelle JP, Rozet E. 2009; Estimation of the stability of parathyroid hormone when stored at -80 degrees C for a long period. Clin J Am Soc Nephrol. 4:1988–92. DOI: 10.2215/CJN.03970609. PMID: 19820128. PMCID: PMC2798870.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Preanalytical Sample Stability between Serum and Ethylenediaminetetraacetic Acid Plasma for the Measurement of Biological Analytes
- Functional Parathyroid Cyst: A case report
- A Study on Serum and Urinary Calcium, Inorganic Phosphorus and Serum Parathyroid Hormone Levels in Patients with Renal Stone
- The Comparison of Parathyroid Hormone Degradation Effect by Various Protease Inhibitors in Blood Specimen
- Study on Serum and Urinary Calcium Level and Serum Parathyroid Hormone in Patients with Urinary Stone