Ann Clin Neurophysiol.
2020 Oct;22(2):104-108. 10.14253/acn.2020.22.2.104.
Intraoperative monitoring of somatosensory and visual evoked potentials for detecting posterior cerebral artery infarction during anteromesial temporal resection
- Affiliations
-
- 1Wonkwang University School of Medicine, Iksan, Korea
- 2Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2511085
- DOI: http://doi.org/10.14253/acn.2020.22.2.104
Abstract
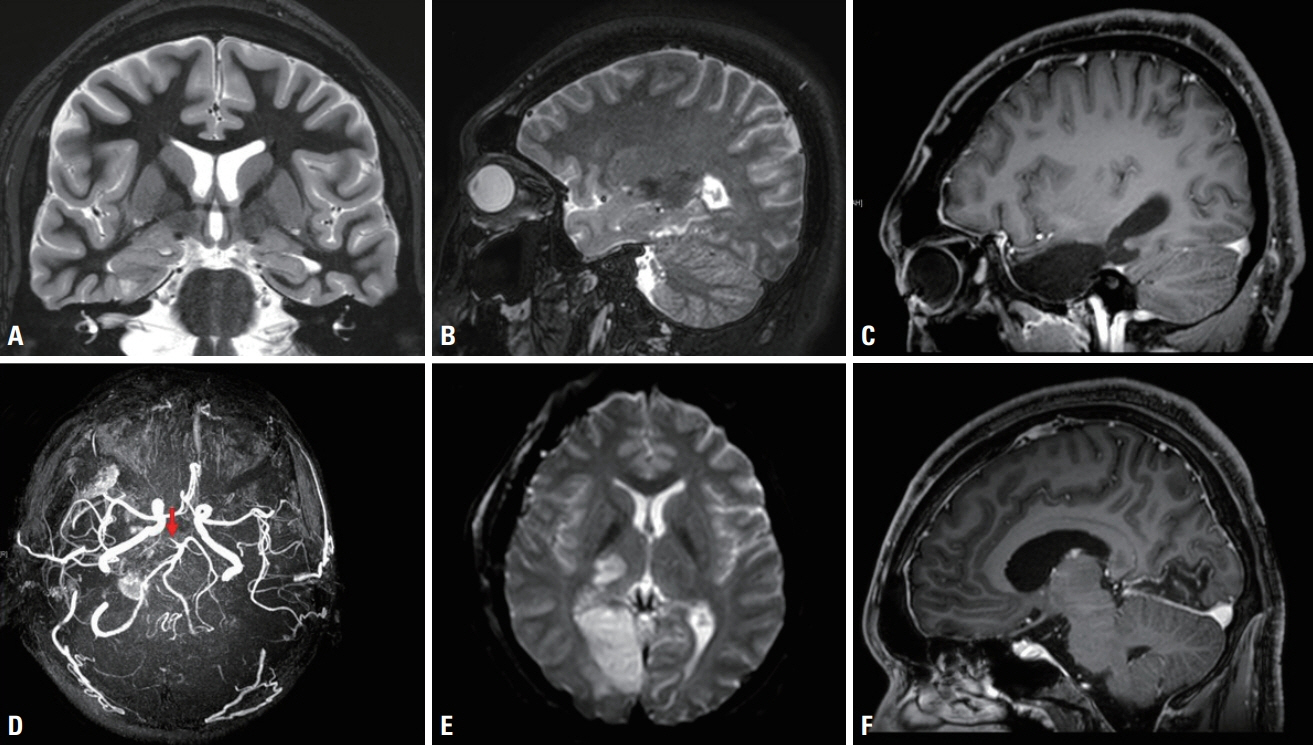
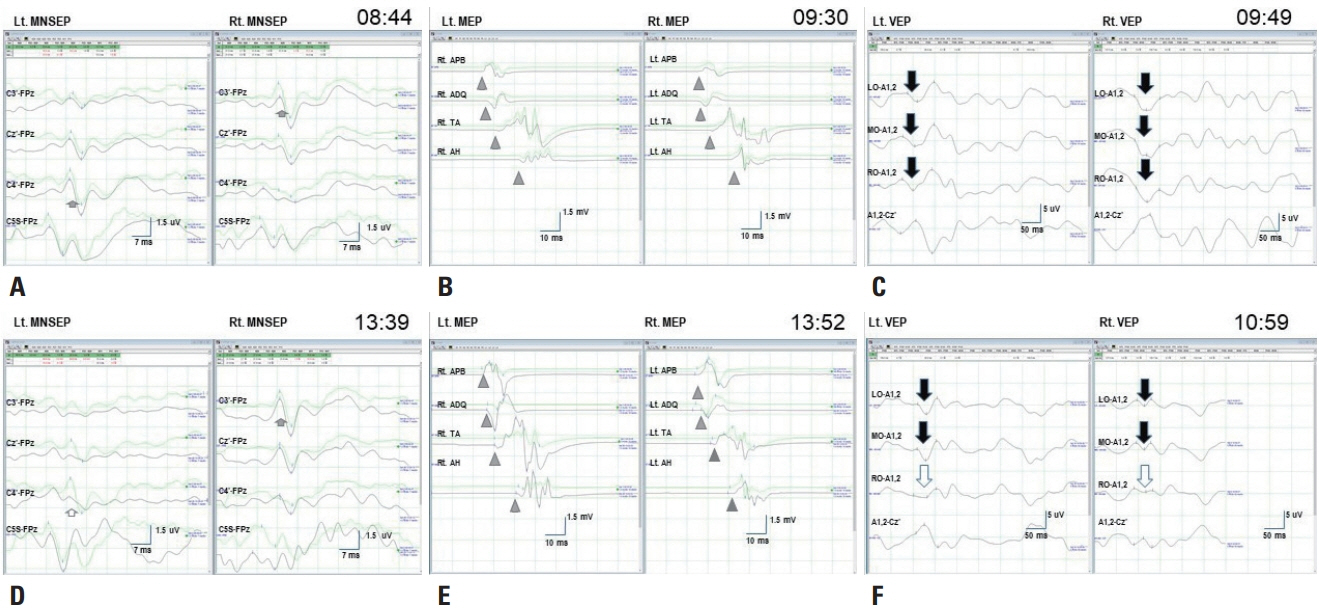
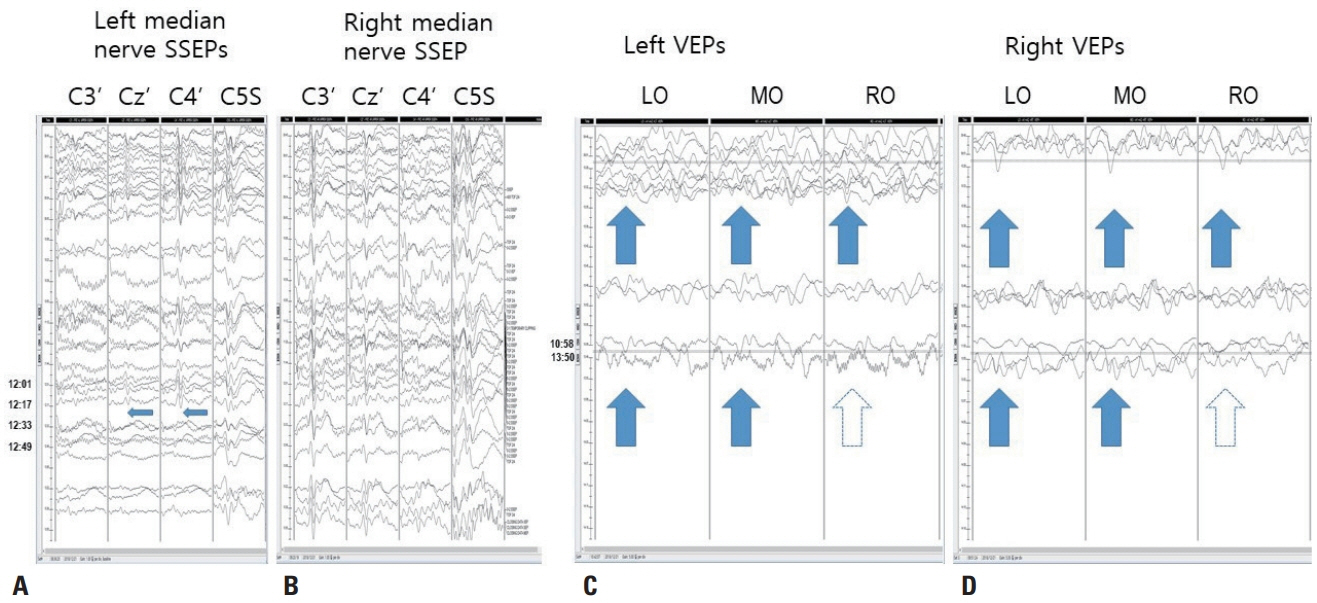
- We performed intraoperative neurophysiological monitoring (INM) during anteromesial temporal resection (AMTR) in a patient with lesional temporal lobe epilepsy. INM revealed a sudden decrease in N20 waves in somatosensory evoked potentials (SSEPs) and poor P100 waves in visual evoked potentials (VEPs). These changes developed after applying electrocoagulation in the right mesial temporal areas. Postoperative brain magnetic resonance imaging demonstrated right thalamic and medial occipital infarctions. SSEPs and VEPs monitoring can be useful for detecting posterior cerebral artery infarction in AMTR.
Keyword
Figure
Reference
-
1. Kim SM, Kim SH, Seo DW, Lee KW. Intraoperative neurophysiologic monitoring: basic principles and recent update. J Korean Med Sci. 2013; 28:1261–1269.
Article2. Koo DL, Lee WG, Hong SC, Seo DW. Clinical usefulness of intraoperative motor-evoked potential monitoring during temporal lobe epilepsy surgery. J Clin Neurol. 2019; 15:285–291.
Article3. Tebo CC, Evins AI, Christos PJ, Kwon J, Schwartz TH. Evolution of cranial epilepsy surgery complication rates: a 32-year systematic review and meta-analysis. J Neurosurg. 2014; 120:1415–1427.
Article4. Gooneratne IK, Mannan S, de Tisi J, Gonzalez JC, McEvoy AW, Miserocchi A, et al. Somatic complications of epilepsy surgery over 25 years at a single center. Epilepsy Res. 2017; 132:70–77.
Article5. Meyer KL, Dempsey RJ, Roy MW, Donaldson DL. Somatosensory evoked potentials as a measure of experimental cerebral ischemia. J Neurosurg. 1985; 62:269–275.
Article6. Thirumala PD, Udesh R, Muralidharan A, Thiagarajan K, Crammond DJ, Chang YF, et al. Diagnostic value of somatosensory-evoked potential monitoring during cerebral aneurysm clipping: a systematic review. World Neurosurg. 2016; 89:672–680.
Article7. Goto T, Tanaka Y, Kodama K, Kusano Y, Sakai K, Hongo K. Loss of visual evoked potential following temporary occlusion of the superior hypophyseal artery during aneurysm clip placement surgery. Case report. J Neurosurg. 2007; 107:865–867.8. Kodama K, Goto T, Sato A, Sakai K, Tanaka Y, Hongo K. Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir (Wien). 2010; 152:643–648.
Article9. Dekeyzer S, De Kock I, Nikoubashman O, Vanden Bossche S, Van Eetvelde R, De Groote J, et al. “Unforgettable” - a pictorial essay on anatomy and pathology of the hippocampus. Insights Imaging. 2017; 8:199–212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Detection of the Internal Capsule Infarction by Intraoperative Neuromonitoring in Mesial Temporal Epilepsy Surgery
- Intraoperative Neurophysiologic Monitoring: Basic Principles and Recent Update
- Monitoring of Somatosensory Evoked Potentials During Intracranial Aneurysm Surgery
- A New Measure for Monitoring Intraoperative Somatosensory Evoked Potentials
- Cerebral somatosensory evoked potentials in children with cerebral palsy