Ann Clin Neurophysiol.
2020 Oct;22(2):51-60. 10.14253/acn.2020.22.2.51.
Muscle pathology in neuromuscular disorders
- Affiliations
-
- 1Department of Neurology, Pusan National University School of Medicine, Yangsan, Korea
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 3Biomedical Research Institute, Pusan National University Yangsan Hospital, Yangsan, Korea
- KMID: 2511079
- DOI: http://doi.org/10.14253/acn.2020.22.2.51
Abstract
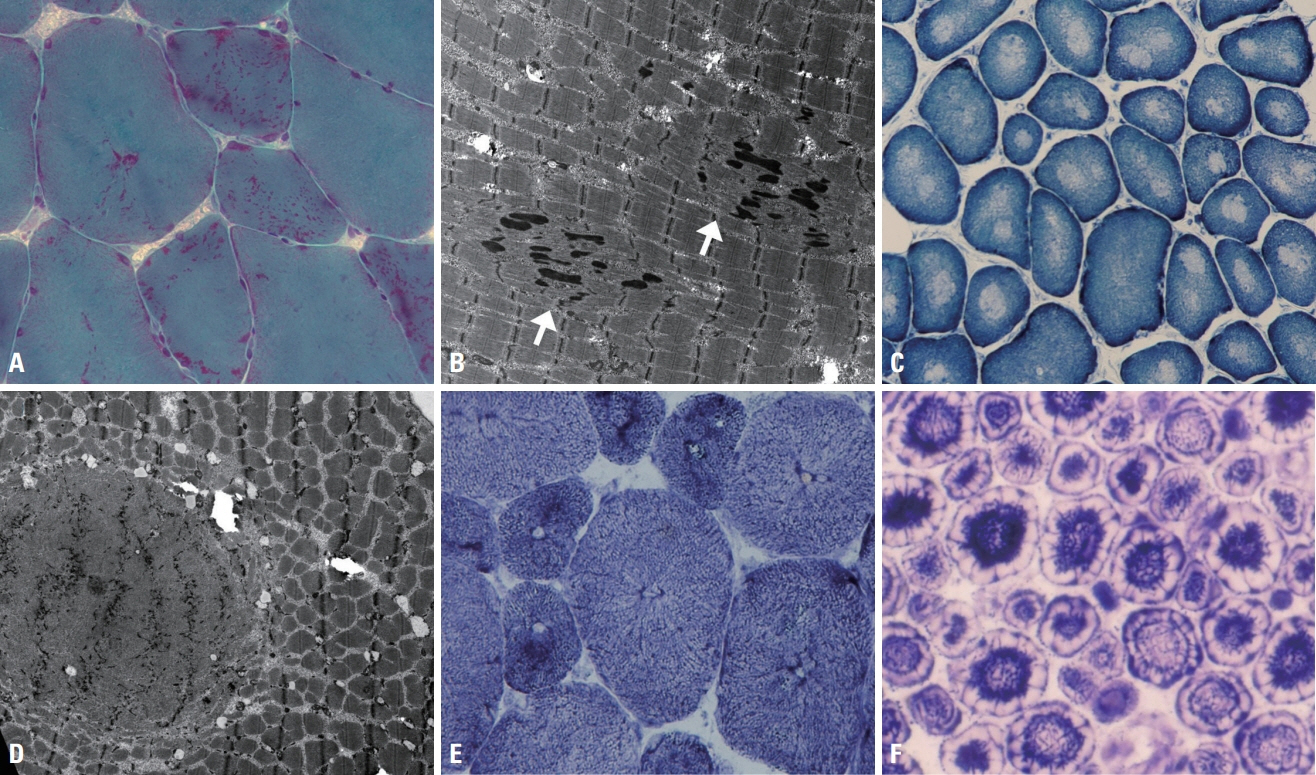
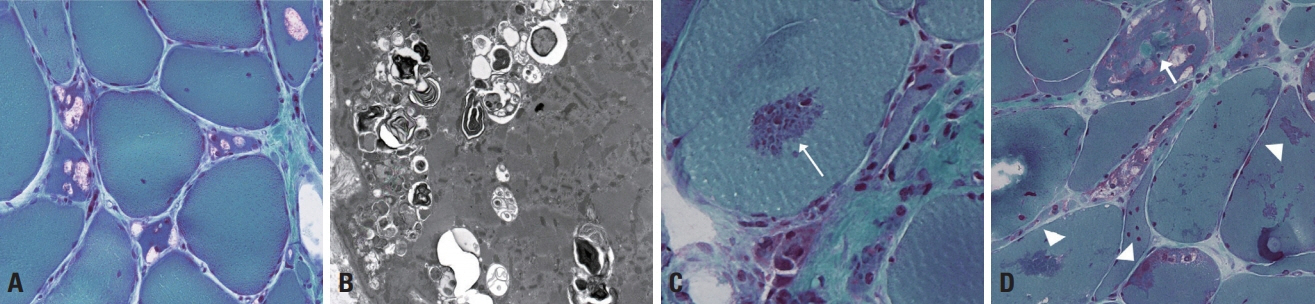
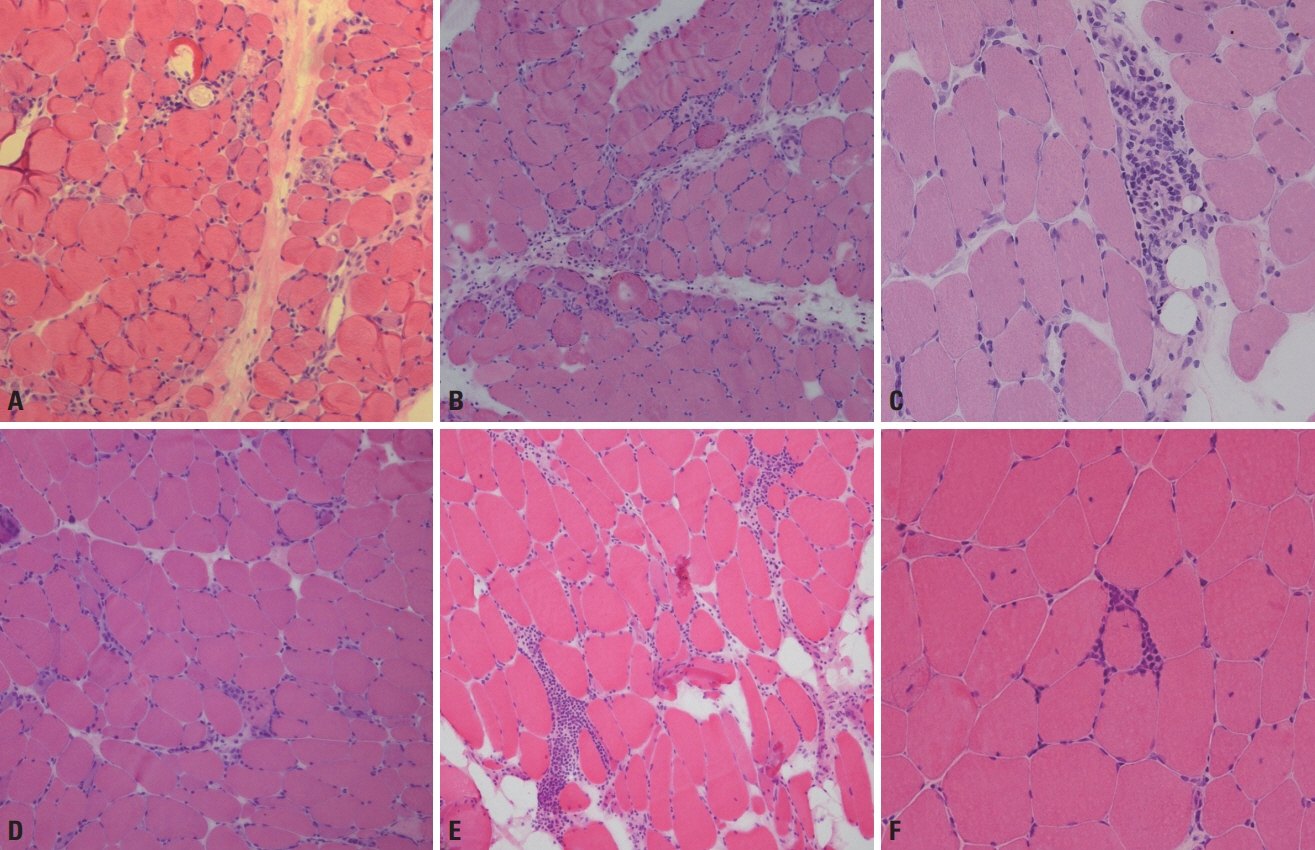
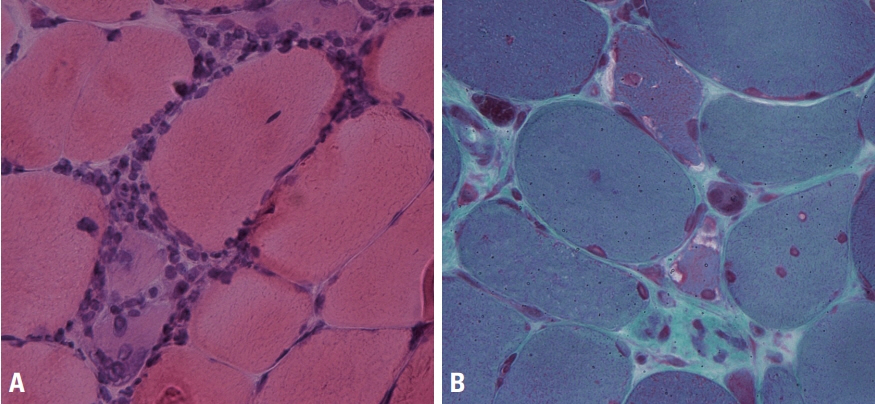
- Muscle pathology findings may guide the diagnosis of neuromuscular disorders since they are helpful for understanding the pathological processes causing muscle weakness and also provide significant clues for the diagnosis of muscle diseases. Recent advances in molecular genetics mean that a muscle biopsy can be omitted when diagnosing inherited muscle diseases. However, the muscle pathology can still play a role in those cases and its findings are also required when diagnosing inflammatory myopathies.
Keyword
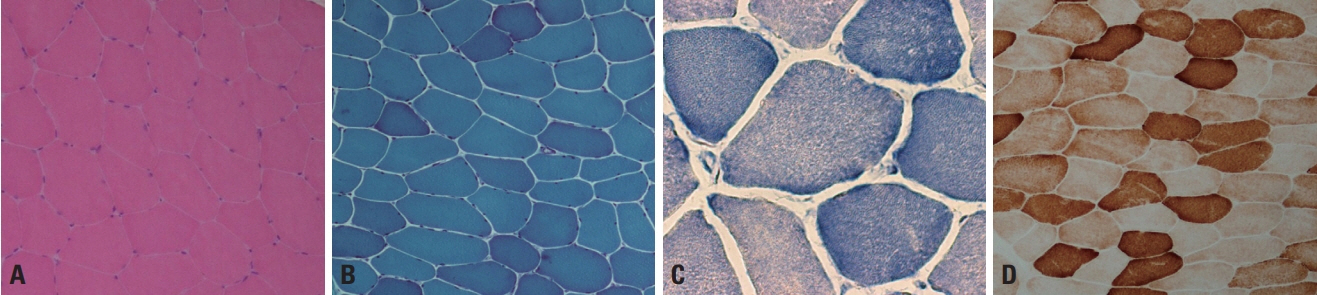
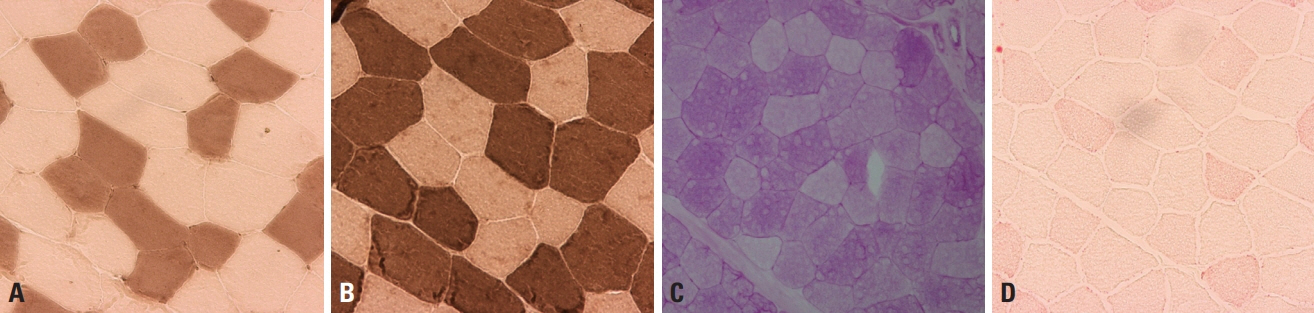
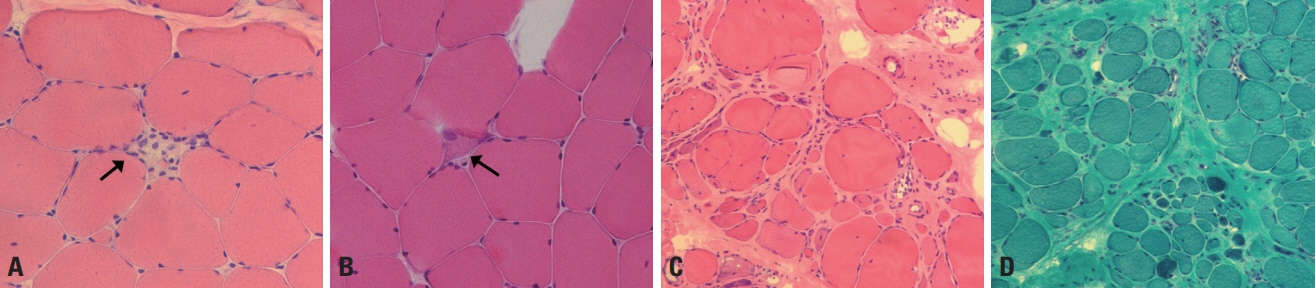
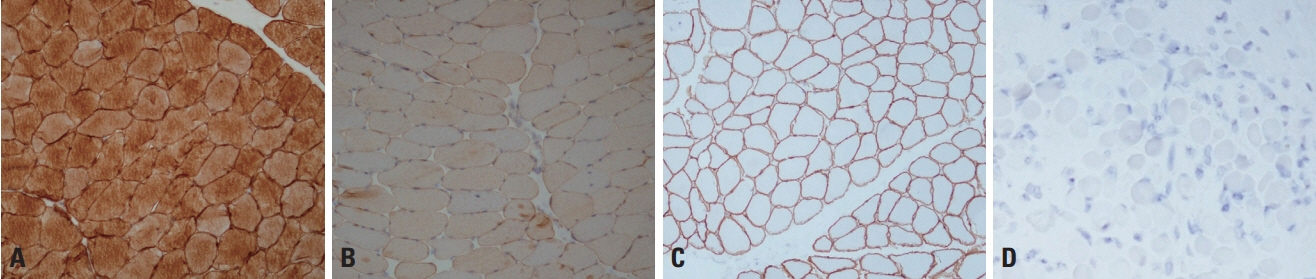
Figure
Reference
-
1. Suzuki S, Uruha A, Suzuki N, Nishino I. Integrated diagnosis project for inflammatory myopathies: an association between autoantibodies and muscle pathology. Autoimmun Rev. 2017; 16:693–700.
Article2. Dubowitz V, Sewry CA, Oldfors A. Normal muscle. In : Dubowitz V, Sewry CA, Oldfors A, Lane R, editors. Muscle biopsy: a practical approach. 4th ed. London: Elsevier;2013. p. 28–54.3. Sewry CA, Goebel HH. General pathology of muscle disease. In : Goebel HH, Sewry CA, Weller RO, editors. Muscle disease: pathology and genetics. 2nd ed. Oxford: Wiley-Blackwell;2013. p. 19–38.4. Dubowitz V, Sewry CA, Oldfors A. Muscular dystrophies and allied disorders I: Duchenne and Becker muscular dystrophy. In : Dubowitz V, Sewry CA, Oldfors A, Lane R, editors. Muscle biopsy: a practical approach. 4th ed. London: Elsevier;2013. p. 250–275.5. Barresi R, Brown SC. Dystrophin and its associated glycoprotein complex. In : Goebel HH, Sewry CA, Weller RO, editors. Muscle disease: pathology and genetics. 2nd ed. Oxford: Wiley-Blackwell;2013. p. 95–101.6. Dubowitz V, Sewry CA. Muscular dystrophies and allied disorders IV: Emery-Dreifuss muscular dystrophy and similar syndromes. In : Dubowitz V, Sewry CA, Oldfors A, Lane R, editors. Muscle biopsy: a practical approach. 4th ed. London: Elsevier;2013. p. 331–344.7. Kim DS. Diagnostic significance of immunohistochemical staining in muscular dystrophy. J Korean Neurol Assoc. 2006; 24:1–13.8. Park YE, Shin JH, Kim HS, Lee CH, Kim DS. Characterization of congenital myopathies at a Korean neuromuscular center. Muscle Nerve. 2018; 58:235–244.
Article9. Ryan MM, Schnell C, Strickland CD, Shield LK, Morgan G, Iannaccone ST, et al. Nemaline myopathy: a clinical study of 143 cases. Ann Neurol. 2001; 50:312–320.
Article10. Lee JM, Lim JG, Shin JH, Park YE, Kim DS. Clinical and genetic diversity of nemaline myopathy from a single neuromuscular center in Korea. J Neurol Sci. 2017; 383:61–68.
Article11. Wattanasirichaigoon D, Swoboda KJ, Takada F, Tong HQ, Lip V, Iannaccone ST, et al. Mutations of the slow muscle alpha-tropomyosin gene, TPM3, are a rare cause of nemaline myopathy. Neurology. 2002; 59:613–617.12. Park YE, Shin JH, Kang B, Lee CH, Kim DS. NEB-related core-rod myopathy with distinct clinical and pathological features. Muscle Nerve. 2016; 53:479–484.
Article13. Naddaf E, Milone M, Kansagra A, Buadi F, Kourelis T. Sporadic late-onset nemaline myopathy: clinical spectrum, survival, and treatment outcomes. Neurology. 2019; 93:e298–e305.14. Park YE, Choi YC, Bae JS, Lee CH, Kim HS, Shin JH, et al. Clinical and pathological features of Korean patients with DNM2-related centronuclear myopathy. J Clin Neurol. 2014; 10:24–31.15. Romero NB, Laforte J. Centronuclear myopathies. In : Goebel HH, Sewry CA, Weller RO, editors. Muscle disease: pathology and genetics. 2nd ed. Oxford: Wiley-Blackwell;2013. p. 134–144.16. Dubowitz V, Sewry CA. Myopathies with vacuoles. In : Dubowitz V, Sewry CA, Oldfors A, Lane R, editors. Muscle biopsy: a practical approach. 4th ed. London: Elsevier;2013. p. 406–422.17. Mair D, Biskup S, Kress W, Abicht A, Brück W, Zechel S, et al. Differential diagnosis of vacuolar myopathies in the NGS era. Brain Pathol. 2020; 30:877–896.
Article18. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975; 292:344–347.19. Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995; 38:705–713.
Article20. Tanboon J, Nishino I. Classification of idiopathic inflammatory myopathies: pathology perspectives. Curr Opin Neurol. 2019; 32:704–714.
Article21. Uruha A, Suzuki S, Suzuki N, Nishino I. Perifascicular necrosis in anti-synthetase syndrome beyond anti-Jo-1. Brain. 2016; 139(Pt 9):e50.
Article