Endocrinol Metab.
2020 Dec;35(4):801-810. 10.3803/EnM.2020.735.
Identification of Novel Genetic Variants Related to Trabecular Bone Score in Community-Dwelling Older Adults
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea
- 3Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, Korea
- 5Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 6Department of Preventive Medicine, Ajou University School of Medicine, Suwon, Korea
- KMID: 2511007
- DOI: http://doi.org/10.3803/EnM.2020.735
Abstract
- Background
As the genetic variants of trabecular bone microarchitecture are not well-understood, we performed a genome-wide association study to identify genetic determinants of bone microarchitecture analyzed by trabecular bone score (TBS).
Methods
TBS-associated genes were discovered in the Ansung cohort (discovery cohort), a community-based rural cohort in Korea, and then validated in the Gene-Environment Interaction and Phenotype (GENIE) cohort (validation cohort), consisting of subjects who underwent health check-up programs. In the discovery cohort, 2,451 participants were investigated for 1.42 million genotyped and imputed markers.
Results
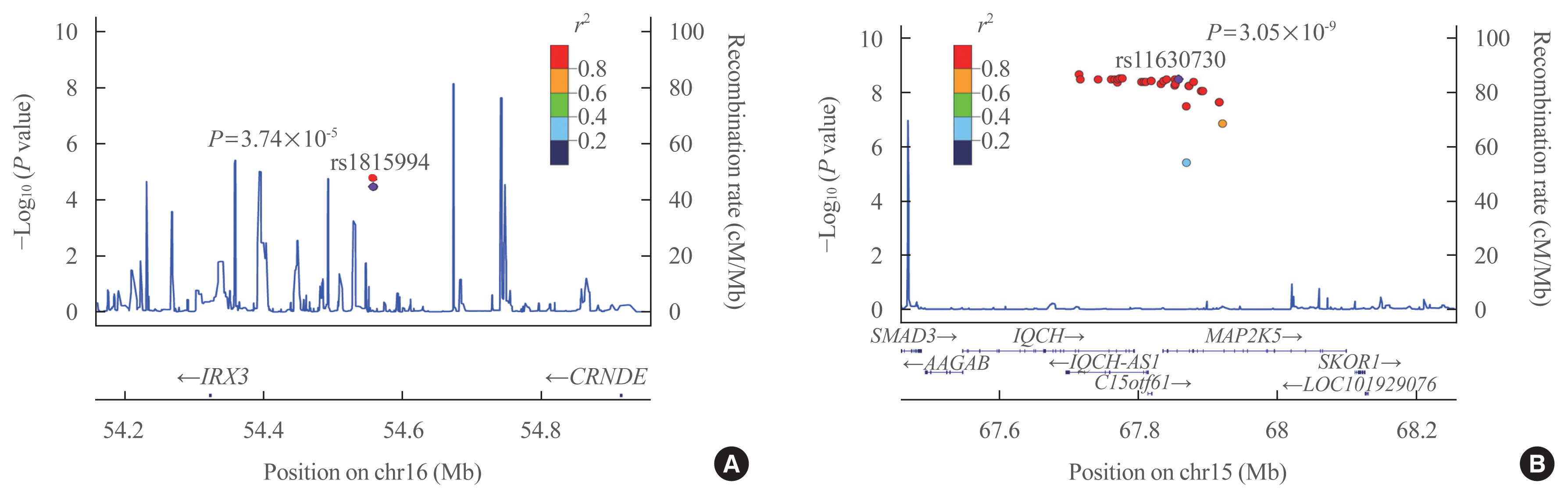
In the validation cohort, identified as significant variants were evaluated in 2,733 participants. An intronic variant in iroquois homeobox 3 (IRX3), rs1815994, was significantly associated with TBS in men (P=3.74E-05 in the discovery cohort, P=0.027 in the validation cohort). Another intronic variant in mitogen-activated protein kinase kinase 5 (MAP2K5), rs11630730, was significantly associated with TBS in women (P=3.05E-09 in the discovery cohort, P=0.041 in the validation cohort). Men with the rs1815994 variant and women with the rs11630730 variant had lower TBS and lumbar spine bone mineral density. The detrimental effects of the rs1815994 variant in men and rs11630730 variant in women were also identified in association analysis (β=–0.0281, β=–0.0465, respectively).
Conclusion
In this study, the rs1815994 near IRX3 in men and rs11630730 near MAP2K5 in women were associated with deterioration of the bone microarchitecture. It is the first study to determine the association of genetic variants with TBS. Further studies are needed to confirm our findings and identify additional variants contributing to the trabecular bone microarchitecture.
Keyword
Figure
Reference
-
1. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008; 42:467–75.
Article2. Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004; 34:195–202.
Article3. Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004; 164:1108–12.
Article4. Seeman E, Delmas PD. Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med. 2006; 354:2250–61.
Article5. Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011; 26:2762–9.
Article6. Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013; 24:77–85.
Article7. Kiel DP, Demissie S, Dupuis J, Lunetta KL, Murabito JM, Karasik D. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet. 2007; 8(Suppl 1):S14.
Article8. Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008; 371:1505–12.
Article9. Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, et al. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008; 358:2355–65.
Article10. Pei YF, Liu L, Liu TL, Yang XL, Zhang H, Wei XT, et al. Joint association analysis identified 18 new loci for bone mineral density. J Bone Miner Res. 2019; 34:1086–94.
Article11. Guo Y, Liu H, Yang TL, Li SM, Li SK, Tian Q, et al. The fat mass and obesity associated gene, FTO, is also associated with osteoporosis phenotypes. PLoS One. 2011; 6:e27312.
Article12. Richards JB, Kavvoura FK, Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, et al. Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med. 2009; 151:528–37.
Article13. Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012; 44:491–501.14. Masi L, Agnusdei D, Bilezikian J, Chappard D, Chapurlat R, Cianferotti L, et al. Taxonomy of rare genetic metabolic bone disorders. Osteoporos Int. 2015; 26:2529–58.
Article15. Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012; 13:576–88.
Article16. Havill LM, Mahaney MC, L Binkley T, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res. 2007; 22:737–46.
Article17. Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009; 41:527–34.
Article18. Hong KW, Kim SS, Kim Y. Genome-wide association study of orthostatic hypotension and supine-standing blood pressure changes in two Korean populations. Genomics Inform. 2013; 11:129–34.
Article19. Lee C, Choe EK, Choi JM, Hwang Y, Lee Y, Park B, et al. Health and Prevention Enhancement (H-PEACE): a retrospective, population-based cohort study conducted at the Seoul National University Hospital Gangnam Center, Korea. BMJ Open. 2018; 8:e019327.
Article20. Kim JH, Choi HJ, Ku EJ, Hong AR, Kim KM, Kim SW, et al. Regional body fat depots differently affect bone microarchitecture in postmenopausal Korean women. Osteoporos Int. 2016; 27:1161–8.
Article21. Sohn S, Chung CK, Han I, Park SB, Kim H. Increased bone mineral density in cervical or thoracic Diffuse Idiopathic Skeletal Hyperostosis (DISH): a case-control study. J Clin Densitom. 2018; 21:68–74.
Article22. Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007; 39:906–13.
Article23. International HapMap Consortium. The international HapMap project. Nature. 2003; 426:789–96.24. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81:559–75.
Article25. Pe’er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008; 32:381–5.
Article26. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014; 67:850–7.27. 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015; 526:68–74.28. Bellefroid EJ, Kobbe A, Gruss P, Pieler T, Gurdon JB, Papalopulu N. Xiro3 encodes a Xenopus homolog of the Drosophila Iroquois genes and functions in neural specification. EMBO J. 1998; 17:191–203.
Article29. Landgraf K, Scholz M, Kovacs P, Kiess W, Korner A. FTO obesity risk variants are linked to adipocyte IRX3 expression and BMI of children: relevance of FTO variants to defend body weight in lean children? PLoS One. 2016; 11:e0161739.30. Scarlett K, Pattabiraman V, Barnett P, Liu D, Anderson LM. The proangiogenic effect of iroquois homeobox transcription factor Irx3 in human microvascular endothelial cells. J Biol Chem. 2015; 290:6303–15.
Article31. Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014; 13:397–406.
Article32. Kiuru M, Solomon J, Ghali B, van der Meulen M, Crystal RG, Hidaka C. Transient overexpression of sonic hedgehog alters the architecture and mechanical properties of trabecular bone. J Bone Miner Res. 2009; 24:1598–607.
Article33. Cain CJ, Gaborit N, Lwin W, Barruet E, Ho S, Bonnard C, et al. Loss of iroquois homeobox transcription factors 3 and 5 in osteoblasts disrupts cranial mineralization. Bone Rep. 2016; 5:86–95.
Article34. Hamamy HA, Teebi AS, Oudjhane K, Shegem NN, Ajlouni KM. Severe hypertelorism, midface prominence, prominent/simple ears, severe myopia, borderline intelligence, and bone fragility in two brothers: new syndrome? Am J Med Genet A. 2007; 143A:229–34.
Article35. Tamamura Y, Katsube K, Mera H, Itokazu M, Wakitani S. Irx3 and Bmp2 regulate mouse mesenchymal cell chondrogenic differentiation in both a Sox9-dependent and -independent manner. J Cell Physiol. 2017; 232:3317–36.
Article36. Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS, et al. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci U S A. 2007; 104:6382–7.
Article37. Kim JM, Yang YS, Park KH, Oh H, Greenblatt MB, Shim JH. The ERK MAPK pathway is essential for skeletal development and homeostasis. Int J Mol Sci. 2019; 20:1803.
Article38. Dinev D, Jordan BW, Neufeld B, Lee JD, Lindemann D, Rapp UR, et al. Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells. EMBO Rep. 2001; 2:829–34.
Article39. Carter EJ, Cosgrove RA, Gonzalez I, Eisemann JH, Lovett FA, Cobb LJ, et al. MEK5 and ERK5 are mediators of the pro-myogenic actions of IGF-2. J Cell Sci. 2009; 122(Pt 17):3104–12.
Article40. Chen TH, Chen CY, Wen HC, Chang CC, Wang HD, Chuu CP, et al. YAP promotes myogenic differentiation via the MEK5-ERK5 pathway. FASEB J. 2017; 31:2963–72.
Article41. Wagner P, Chapurlat R, Ecochard R, Szulc P. Low muscle strength and mass is associated with the accelerated decline of bone microarchitecture at the distal radius in older men: the prospective STRAMBO study. J Bone Miner Res. 2018; 33:1630–40.
Article42. Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol. 2001; 13:600–3.
Article43. Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006; 16:51–9.44. Del Mare S, Kurek KC, Stein GS, Lian JB, Aqeilan RI. Role of the WWOX tumor suppressor gene in bone homeostasis and the pathogenesis of osteosarcoma. Am J Cancer Res. 2011; 1:585–94.45. Kurek KC, Del Mare S, Salah Z, Abdeen S, Sadiq H, Lee SH, et al. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res. 2010; 70:5577–86.
Article46. Leslie WD, Aubry-Rozier B, Lamy O, Hans D; Manitoba Bone Density Program. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013; 98:602–9.
Article47. Harvey NC, Gluer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015; 78:216–24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Linear Association between Frailty as Assessed by the Kihon Checklist and Quality of Life in Community-Dwelling Older Adults: A Cross-Sectional Population-Based Study
- Comparison of Comprehensive Health Status and Health-related Quality of Life between Institutionalized Older Adults and Community Dwelling Older Adults
- Influence of Advance Directives' Self-efficacy of Community-dwelling Older Adults on the Completion of Advance Directives: Mediating the Effect of Intention for Advance Directives: A Cross-Sectional Study
- Sleep Patterns of Older Residents in Long-Term-Care Facilities: A Comparison with Older Adults in Home-Care Services and Community-Dwelling Older Adults
- Factors Contributing to Low Weight in Community-Dwelling Older Adults