Cancer Res Treat.
2021 Jan;53(1):162-171. 10.4143/crt.2020.704.
Spatial Distribution and Prognostic Implications of Tumor-Infiltrating FoxP3- CD4+ T Cells in Biliary Tract Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea
- 3Department of Pathology, Asan Medical Center, Seoul, University of Ulsan College of Medicine, Korea
- 4Department of Surgery, Asan Medical Center, Seoul, University of Ulsan College of Medicine, Korea
- KMID: 2510658
- DOI: http://doi.org/10.4143/crt.2020.704
Abstract
- Purpose
The clinical implications of tumor-infiltrating T cell subsets and their spatial distribution in biliary tract cancer (BTC) patients treated with gemcitabine plus cisplatin were investigated.
Materials and Methods
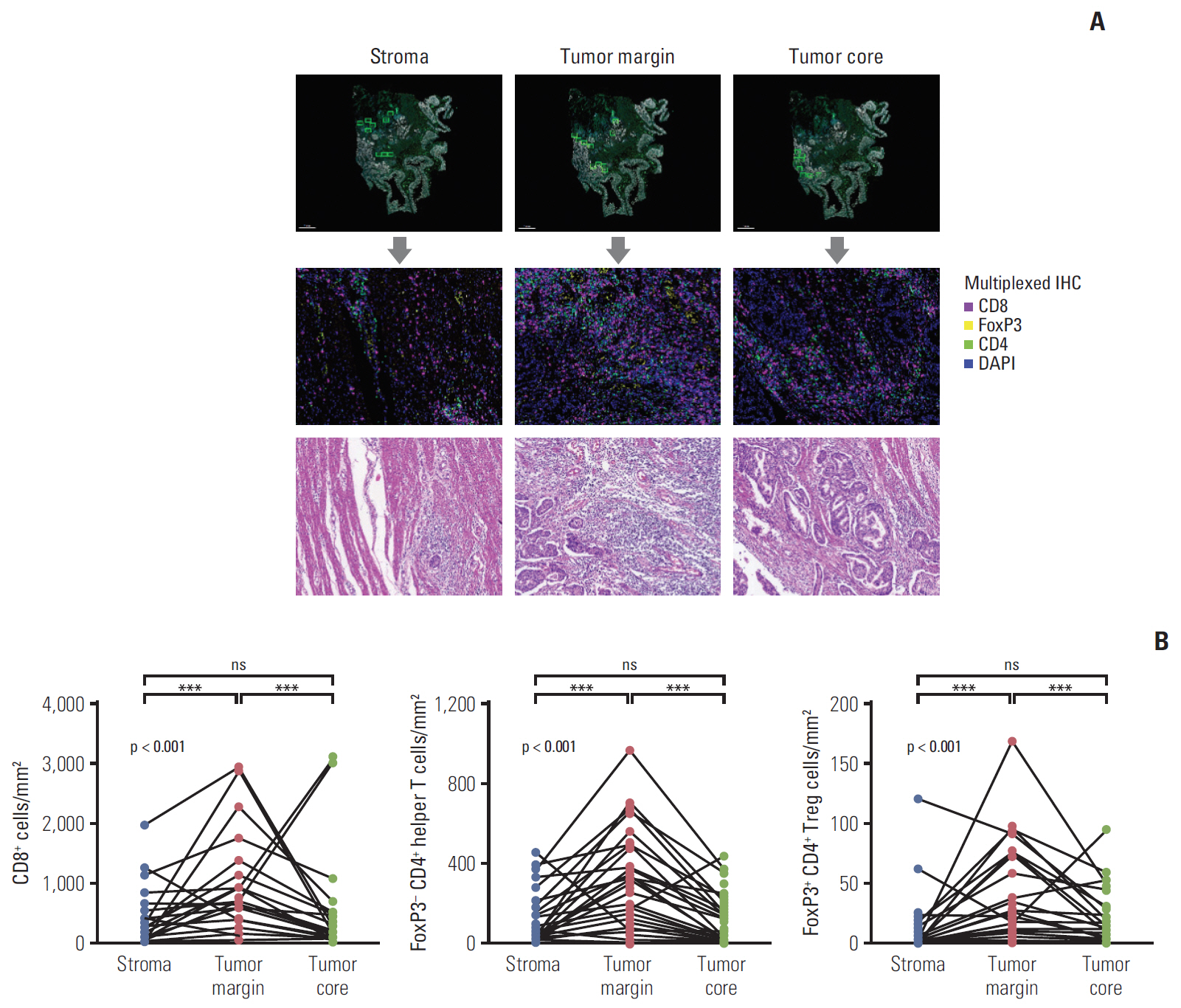
A total of 52 BTC patients treated with palliative gemcitabine plus cisplatin were included. Multiplexed immunohistochemistry was performed on tumor tissues, and immune infiltrates were separately analyzed for the stroma, tumor margin, and tumor core.
Results
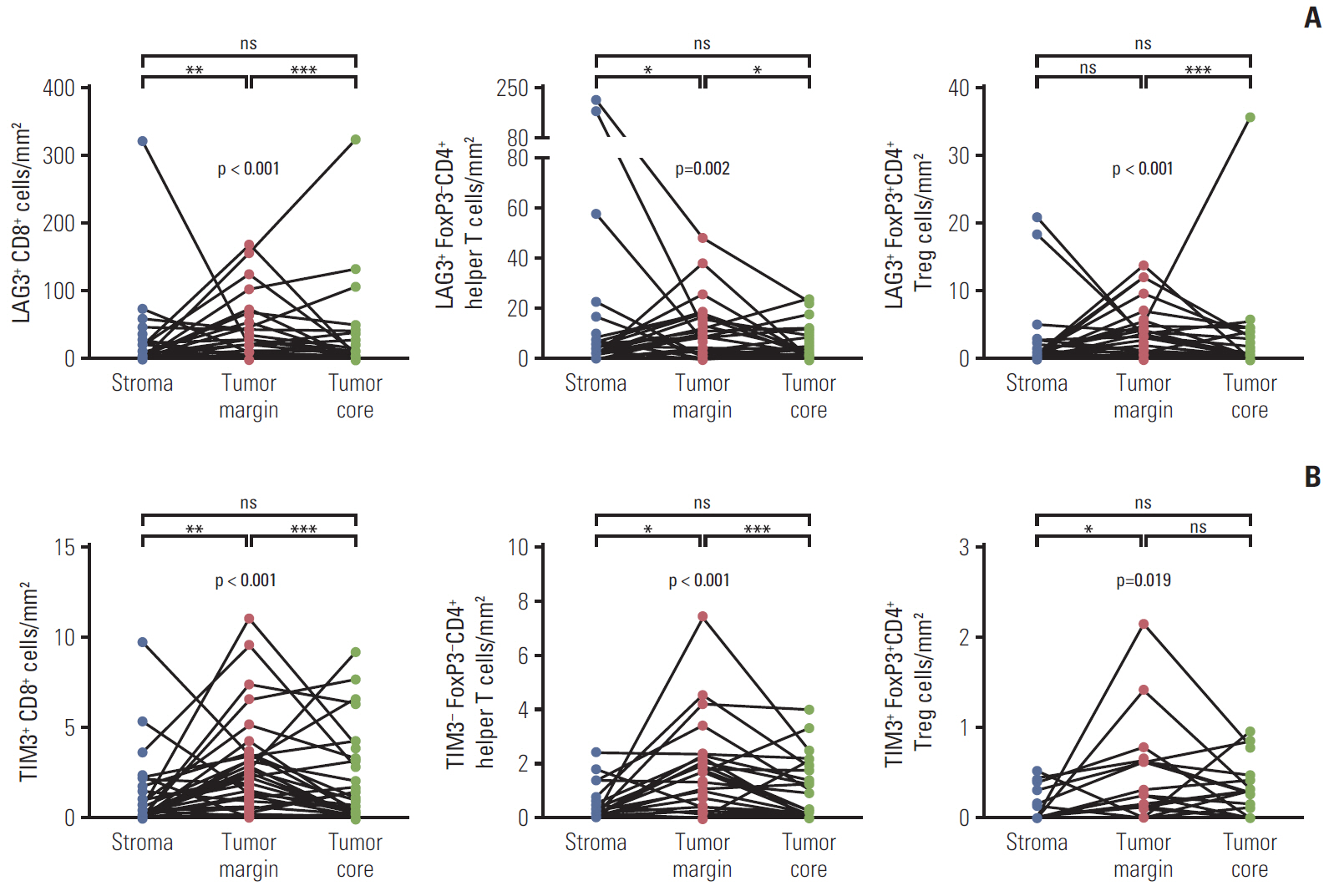
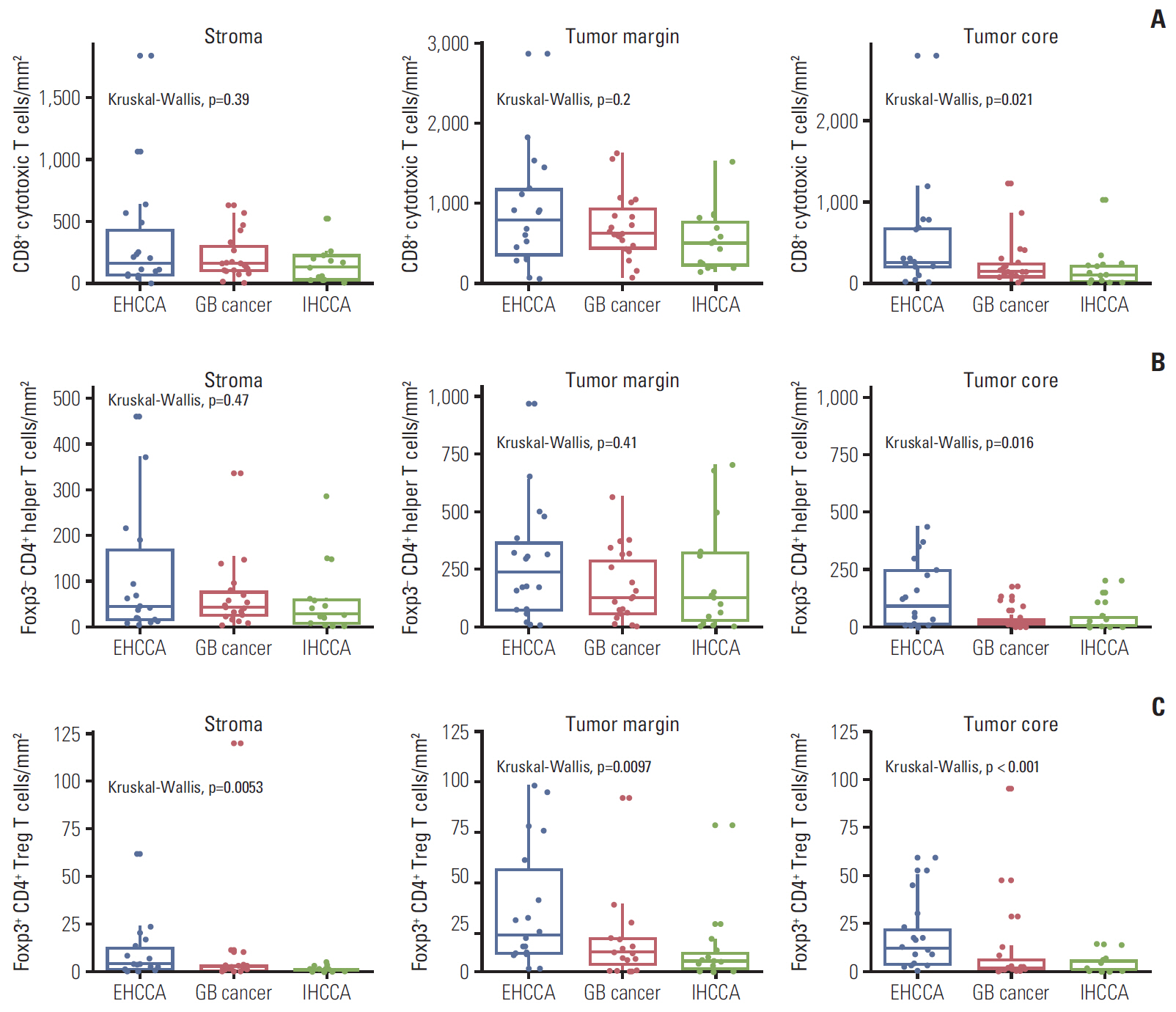
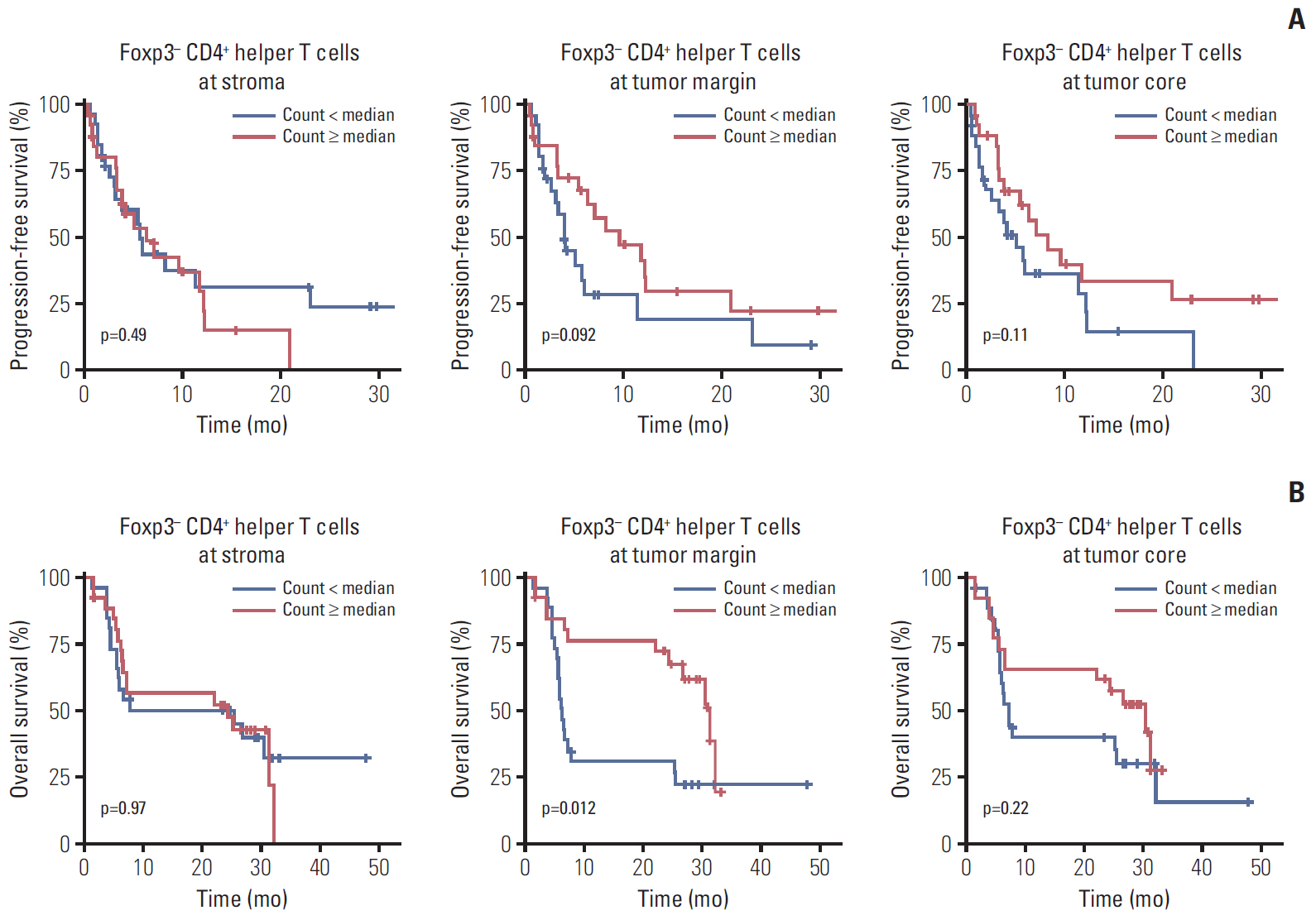
The density of CD8+ T cells, FoxP3- CD4+ helper T cells, and FoxP3+ CD4+ regulatory T cells was significantly higher in the tumor margin than in the stroma and tumor core. The density of LAG3- or TIM3-expressing CD8+ T cell and FoxP3- CD4+ helper T cell infiltrates was also higher in the tumor margin. In extrahepatic cholangiocarcinoma, there was a higher density of T cell subsets in the tumor core and regulatory T cells in all regions. A high density of FoxP3- CD4+ helper T cells in the tumor margin showed a trend toward better progression-free survival (PFS) (p=0.092) and significantly better overall survival (OS) (p=0.012). In multivariate analyses, a high density of FoxP3- CD4+ helper T cells in the tumor margin was independently associated with favorable PFS and OS.
Conclusion
The tumor margin is the major site for the active infiltration of T cell subsets with higher levels of LAG3 and TIM3 expression in BTC. The density of tumor margin-infiltrating FoxP3- CD4+ helper T cells may be associated with clinical outcomes in BTC patients treated with gemcitabine plus cisplatin.
Figure
Reference
-
References
1. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article2. Chae H, Kim D, Yoo C, Kim KP, Jeong JH, Chang HM, et al. Therapeutic relevance of targeted sequencing in management of patients with advanced biliary tract cancer: DNA damage repair gene mutations as a predictive biomarker. Eur J Cancer. 2019; 120:31–9.
Article3. Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapyrefractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020; 21:796–807.
Article4. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020; 21:671–84.
Article5. Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020; 8:e000564.6. Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. 2019; 4:611–21.
Article7. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020; 147:2190–8.8. Kang J, Jeong JH, Hwang HS, Lee SS, Park DH, Oh DW, et al. Efficacy and safety of pembrolizumab in patients with refractory advanced biliary tract cancer: tumor proportion score as a potential biomarker for response. Cancer Res Treat. 2020; 52:594–603.
Article9. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020; 20:651–68.
Article10. Borst J, Ahrends T, Babala N, Melief CJ, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018; 18:635–47.
Article11. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression: implications for anticancer therapy. Nat Rev Clin Oncol. 2019; 16:356–71.12. Kitano Y, Okabe H, Yamashita YI, Nakagawa S, Saito Y, Umezaki N, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018; 118:171–80.
Article13. Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013; 109:2665–74.
Article14. Massi D, Rulli E, Cossa M, Valeri B, Rodolfo M, Merelli B, et al. The density and spatial tissue distribution of CD8(+) and CD163(+) immune cells predict response and outcome in melanoma patients receiving MAPK inhibitors. J Immunother Cancer. 2019; 7:308.
Article15. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515:568–71.
Article16. Godet Y, Fabre E, Dosset M, Lamuraglia M, Levionnois E, Ravel P, et al. Analysis of spontaneous tumor-specific CD4 T-cell immunity in lung cancer using promiscuous HLA-DR telomerase-derived epitopes: potential synergistic effect with chemotherapy response. Clin Cancer Res. 2012; 18:2943–53.
Article17. Yuan Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb Perspect Med. 2016; 6:a026583.
Article18. Heindl A, Nawaz S, Yuan Y. Mapping spatial heterogeneity in the tumor microenvironment: a new era for digital pathology. Lab Invest. 2015; 95:377–84.
Article19. Kather JN, Suarez-Carmona M, Charoentong P, Weis CA, Hirsch D, Bankhead P, et al. Topography of cancer-associated immune cells in human solid tumors. Elife. 2018; 7:e36967.
Article20. Kim BJ, Hyung J, Yoo C, Kim KP, Park SJ, Lee SS, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017; 80:209–15.
Article21. Christgen M, von Ahsen S, Christgen H, Langer F, Kreipe H. The region-of-interest size impacts on Ki67 quantification by computer-assisted image analysis in breast cancer. Hum Pathol. 2015; 46:1341–9.
Article22. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. 2018; 48:812–30.23. Kim HD, Song GW, Park S, Jung MK, Kim MH, Kang HJ, et al. Association between expression level of PD1 by tumor-infiltrating CD8(+) T cells and features of hepatocellular carcinoma. Gastroenterology. 2018; 155:1936–50.24. Kim HD, Park S, Jeong S, Lee YJ, Lee H, Kim CG, et al. 4-1BB delineates distinct activation status of exhausted tumor-infiltrating CD8(+) T cells in hepatocellular carcinoma. Hepatology. 2020; 71:955–71.25. Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, et al. Intratumoral CD4(+) T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell. 2020; 181:1612–25.
Article26. Takagi S, Miyagawa S, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum Pathol. 2004; 35:881–6.
Article27. Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012; 61:2343–56.
Article28. Ding ZC, Zhou G. Cytotoxic chemotherapy and CD4+ effector T cells: an emerging alliance for durable antitumor effects. Clin Dev Immunol. 2012; 2012:890178.29. Soh JS, Jo SI, Lee H, Do EJ, Hwang SW, Park SH, et al. Immunoprofiling of colitis-associated and sporadic colorectal cancer and its clinical significance. Sci Rep. 2019; 9:6833.
Article30. Hofman P, Badoual C, Henderson F, Berland L, Hamila M, Long-Mira E, et al. Multiplexed immunohistochemistry for molecular and immune profiling in lung cancer-just about ready for prime-time? Cancers (Basel). 2019; 11:283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Zonal Difference and Prognostic Significance of Foxp3 Regulatory T Cell Infiltration in Breast Cancer
- Contrasting Prognostic Effects of Tumor-Infiltrating Lymphocyte Density in Cardia and Non-cardia Gastric Adenocarcinomas
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Significance of Foxp3 Positive Regulatory T Cell and Tumor Infiltrating T Lymphocyte in Triple Negative Breast Cancer