Anat Cell Biol.
2020 Dec;53(4):481-492. 10.5115/acb.20.085.
A rice bran phytochemical, cyanidin 3-glucoside, inhibits the progression of PC3 prostate cancer cell
- Affiliations
-
- 1Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, Thailand
- 2Department of Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- KMID: 2509694
- DOI: http://doi.org/10.5115/acb.20.085
Abstract
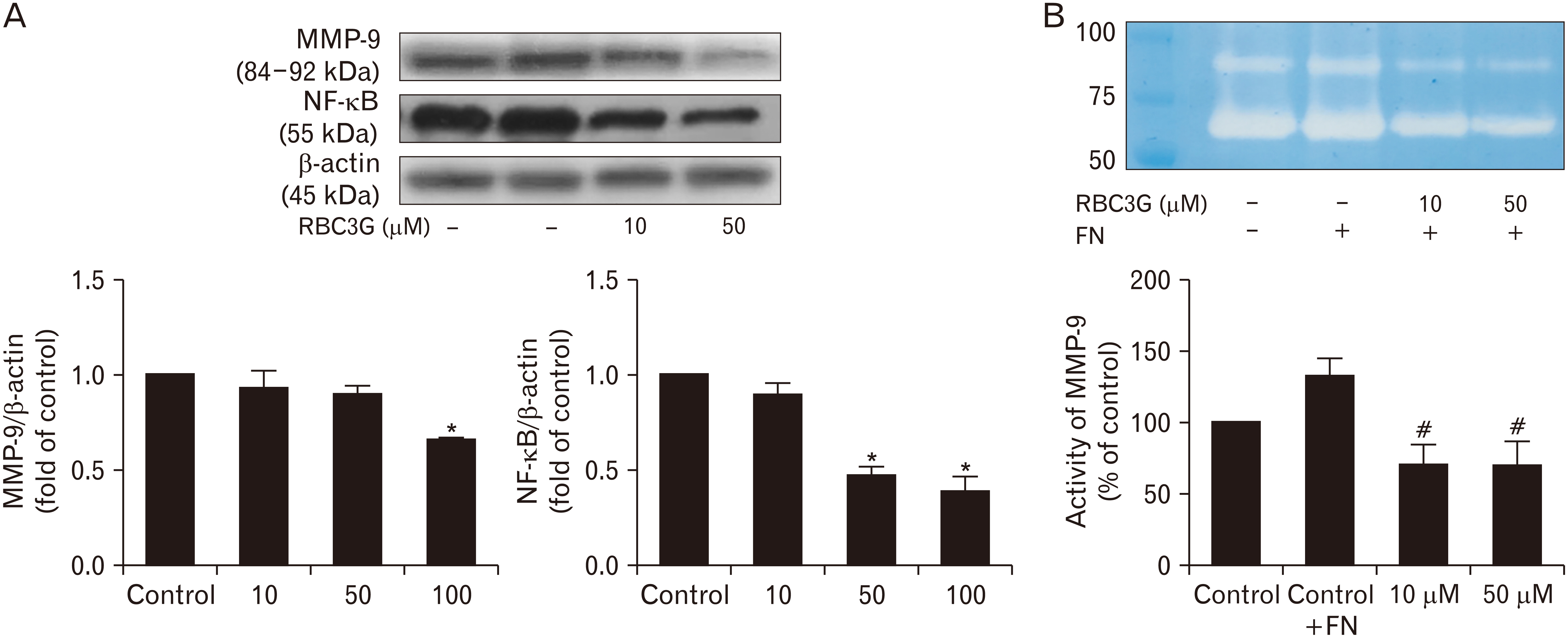
- Prostate cancer is one of the high incidences and the most invasive cancer that is also highly resistant to chemotherapy. Currently, several natural products have been considering using as the supplements for anti-cancer therapy. This study aims to identify the potential active anti-cancer ingredients in the bran extracts of the native Thai rice (Luempua cultivar). Rice bran fraction enriched in anthocyanins was successively isolated and processed until the major purified compound obtained. The sub-fractions and the purified, rice bran, cyanidin 3-glucoside (RBC3G), were studied for biological effects (cell viability, migration, and invasion assays) on human prostatic cancer (PC3) cells using immunohistochemicalstaining and immuno-blotting approaches. The sub-fractions and the purified RBC3G inhibited epithelial mesenchymal transition (EMT) characteristics of PC3 cells by blocking the expression of several cytoskeletal associate proteins in a concentration dependent manner, leading to decreasing of the cancer cell motility. RBC3G reduced the expression of Smad/Snail signaling molecules but enhanced the expression of cell surface protein, E-cadherin, leading to a delay tumor transformation. The RBC3G also inhibited matrix metalloproteinase-9 and nuclear factor-kappa B expression levels and the enzymes activity in PC3 cells, leading to a slow cell migration/invasion process. The results suggested that RBC3G blunt and/ or delay the progressive cancer cell behaviors by inhibit EMT through Smad signaling pathway(s) mediating Snail/E-cadherin expression.
Figure
Reference
-
References
1. Matchett MD, MacKinnon SL, Sweeney MI, Gottschall-Pass KT, Hurta RA. 2006; Inhibition of matrix metalloproteinase activity in DU145 human prostate cancer cells by flavonoids from lowbush blueberry (Vaccinium angustifolium): possible roles for protein kinase C and mitogen-activated protein-kinase-mediated events. J Nutr Biochem. 17:117–25. DOI: 10.1016/j.jnutbio.2005.05.014. PMID: 16111875.2. Lu JN, Lee WS, Kim MJ, Yun JW, Jung JH, Yi SM, Jeong JH, Kim HJ, Choi YH, Kim GS, Ryu CH, Shin SC. 2014; The inhibitory effect of anthocyanins on Akt on invasion and epithelial-mesenchymal transition is not associated with the anti-EGFR effect of the anthocyanins. Int J Oncol. 44:1756–66. DOI: 10.3892/ijo.2014.2315. PMID: 24585214.
Article3. Somintara S, Leardkamolkarn V, Suttiarporn P, Mahatheeranont S. 2016; Anti-tumor and immune enhancing activities of rice bran gramisterol on acute myelogenous leukemia. PLoS One. 11:e0146869. DOI: 10.1371/journal.pone.0146869. PMID: 26752299. PMCID: PMC4709086.
Article4. Srisuwan S, Arkaravichien T, Mahatheeranont S, Puangsombat P, Seekhaw P, Noenplab ANL, Sattayasai J. 2015; Effects of aqueous extract of unpolished dark purple glutinous rice, Var Luem Pua, on ROS in SK-N-SH cells and scopolamine-induced memory deficit in mice. Trop J Pharm Res. 14:1635–41.
Article5. Pitija K, Nakornriab M, Sriseadka T, Vanavichit A, Wongpornchai S. 2013; Anthocyanin content and antioxidant capacity in bran extracts of some Thai black rice varieties. Int J Food Sci Tech. 48:300–8. DOI: 10.1111/j.1365-2621.2012.03187.x.
Article6. Wang LS, Stoner GD. 2008; Anthocyanins and their role in cancer prevention. Cancer Lett. 269:281–90. DOI: 10.1016/j.canlet.2008.05.020. PMID: 18571839. PMCID: PMC2582525.
Article7. Chen PN, Kuo WH, Chiang CL, Chiou HL, Hsieh YS, Chu SC. 2006; Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem Biol Interact. 163:218–29. DOI: 10.1016/j.cbi.2006.08.003. PMID: 16970933.
Article8. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. 2016; Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 66:271–89. DOI: 10.3322/caac.21349. PMID: 27253694.
Article9. Lin HC, Lin JY. 2016; Immune cell-conditioned media suppress prostate cancer PC-3 cell growth correlating with decreased proinflammatory/anti-inflammatory cytokine ratios in the media using 5 selected crude polysaccharides. Integr Cancer Ther. 15:NP13–25. DOI: 10.1177/1534735415627923. PMID: 27130724. PMCID: PMC5739154.
Article10. Seidi K, Jahanban-Esfahlan R, Abasi M, Abbasi MM. 2016; Anti tumoral properties of Punica granatum (Pomegranate) seed extract in different human cancer cells. Asian Pac J Cancer Prev. 17:1119–22. DOI: 10.7314/APJCP.2016.17.3.1119. PMID: 27039735.
Article11. Modaeinama S, Abasi M, Abbasi MM, Jahanban-Esfahlan R. 2015; Anti tumoral properties of Punica granatum (Pomegranate) peel extract on different human cancer cells. Asian Pac J Cancer Prev. 16:5697–701. DOI: 10.7314/APJCP.2015.16.14.5697. PMID: 26320438.
Article12. Peng CC, Peng CH, Chen KC, Hsieh CL, Peng RY. 2011; The aqueous soluble polyphenolic fraction of psidium guajava leaves exhibits potent anti-angiogenesis and anti-migration actions on DU145 cells. Evid Based Complement Alternat Med. 2011:219069. DOI: 10.1093/ecam/neq005. PMID: 21799674. PMCID: PMC3135903.13. Chen KC, Peng CC, Chiu WT, Cheng YT, Huang GT, Hsieh CL, Peng RY. 2010; Action mechanism and signal pathways of Psidium guajava L. aqueous extract in killing prostate cancer LNCaP cells. Nutr Cancer. 62:260–70. DOI: 10.1080/01635580903407130. PMID: 20099201.14. Albrecht M, Jiang W, Kumi-Diaka J, Lansky EP, Gommersall LM, Patel A, Mansel RE, Neeman I, Geldof AA, Campbell MJ. 2004; Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food. 7:274–83. DOI: 10.1089/jmf.2004.7.274. PMID: 15383219.
Article15. Salehi B, Fokou PVT, Yamthe LRT, Tali BT, Adetunji CO, Rahavian A, Mudau FN, Martorell M, Setzer WN, Rodrigues CF, Martins N, Cho WC, Sharifi-Rad J. 2019; Phytochemicals in prostate cancer: from bioactive molecules to upcoming therapeutic agents. Nutrients. 11:1483. DOI: 10.3390/nu11071483. PMID: 31261861. PMCID: PMC6683070.
Article16. Zhang ZH, Xie DD, Xu S, Xia MZ, Zhang ZQ, Geng H, Chen L, Wang DM, Wei W, Yu DX, Xu DX. 2017; Total glucosides of paeony inhibits lipopolysaccharide-induced proliferation, migration and invasion in androgen insensitive prostate cancer cells. PLoS One. 12:e0182584. DOI: 10.1371/journal.pone.0182584. PMID: 28783760. PMCID: PMC5544245.
Article17. Sorrenti V, Vanella L, Acquaviva R, Cardile V, Giofrè S, Di Giacomo C. 2015; Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int J Oncol. 47:1303–10. DOI: 10.3892/ijo.2015.3130. PMID: 26315029.
Article18. Roviello GN, Iannitti R, Roviello V, Palumbo R, Simonyan H, Vicidomini C. 2017; Synthesis and biological evaluation of a novel Amadori compound. Amino Acids. 49:327–35. DOI: 10.1007/s00726-016-2363-4. PMID: 27864693.
Article19. Roviello GN, Iannitti R, Palumbo R, Simonyan H, Vicidomini C, Roviello V. 2017; Lac-L-TTA, a novel lactose-based amino acid-sugar conjugate for anti-metastatic applications. Amino Acids. 49:1347–53. DOI: 10.1007/s00726-017-2433-2. PMID: 28478584.
Article20. Carella A, Roviello V, Iannitti R, Palumbo R, La Manna S, Marasco D, Trifuoggi M, Diana R, Roviello GN. 2019; Evaluating the biological properties of synthetic 4-nitrophenyl functionalized benzofuran derivatives with telomeric DNA binding and antiproliferative activities. Int J Biol Macromol. 121:77–88. DOI: 10.1016/j.ijbiomac.2018.09.153. PMID: 30261256.
Article21. Voulgari A, Pintzas A. 2009; Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 1796:75–90. DOI: 10.1016/j.bbcan.2009.03.002. PMID: 19306912.
Article22. Kim DH, Xing T, Yang Z, Dudek R, Lu Q, Chen YH. 2017; Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 7:1. DOI: 10.3390/jcm7010001. PMID: 29271928. PMCID: PMC5791009.
Article23. Lamouille S, Xu J, Derynck R. 2014; Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 15:178–96. DOI: 10.1038/nrm3758. PMID: 24556840. PMCID: PMC4240281.
Article24. Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. 2011; Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 30:1436–48. DOI: 10.1038/onc.2010.509. PMID: 21057535.
Article25. Tran NL, Nagle RB, Cress AE, Heimark RL. 1999; N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion withStromal cells. Am J Pathol. 155:787–98. DOI: 10.1016/S0002-9440(10)65177-2. PMID: 10487836. PMCID: PMC1866912.26. Valenta T, Hausmann G, Basler K. 2012; The many faces and functions of β-catenin. EMBO J. 31:2714–36. DOI: 10.1038/emboj.2012.150. PMID: 22617422. PMCID: PMC3380220.
Article27. Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S, Liang T. 2013; Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 34:962–73. DOI: 10.1093/carcin/bgt027. PMID: 23358852.
Article28. Lim J, Thiery JP. 2012; Epithelial-mesenchymal transitions: insights from development. Development. 139:3471–86. DOI: 10.1242/dev.071209. PMID: 22949611.
Article29. Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. 2008; A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9:582–9. DOI: 10.1038/embor.2008.74. PMID: 18483486. PMCID: PMC2396950.
Article30. Clevers H. 2006; Wnt/beta-catenin signaling in development and disease. Cell. 127:469–80. DOI: 10.1016/j.cell.2006.10.018. PMID: 17081971.31. Tu B, Peng ZX, Fan QM, Du L, Yan W, Tang TT. 2014; Osteosarcoma cells promote the production of pro-tumor cytokines in mesenchymal stem cells by inhibiting their osteogenic differentiation through the TGF-β/Smad2/3 pathway. Exp Cell Res. 320:164–73. DOI: 10.1016/j.yexcr.2013.10.013. PMID: 24183998.
Article32. Wang Y, Shi J, Chai K, Ying X, Zhou BP. 2013; The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 13:963–72. DOI: 10.2174/15680096113136660102. PMID: 24168186. PMCID: PMC4004763.
Article33. Ding M, Feng R, Wang SY, Bowman L, Lu Y, Qian Y, Castranova V, Jiang BH, Shi X. 2006; Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J Biol Chem. 281:17359–68. DOI: 10.1074/jbc.M600861200. PMID: 16618699.
Article34. Cho E, Chung EY, Jang HY, Hong OY, Chae HS, Jeong YJ, Kim SY, Kim BS, Yoo DJ, Kim JS, Park KH. 2017; Anti-cancer effect of cyanidin-3-glucoside from mulberry via caspase-3 cleavage and DNA fragmentation in vitro and in vivo. Anticancer Agents Med Chem. 17:1519–25. DOI: 10.2174/1871520617666170327152026. PMID: 28356020.
Article35. Chen PN, Chu SC, Chiou HL, Chiang CL, Yang SF, Hsieh YS. 2005; Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr Cancer. 53:232–43. DOI: 10.1207/s15327914nc5302_12. PMID: 16573384.36. Lin BW, Gong CC, Song HF, Cui YY. 2017; Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol. 174:1226–43. DOI: 10.1111/bph.13627. PMID: 27646173. PMCID: PMC5429338.
Article37. Yun CY, Choi H, You YJ, Yang JY, Baek JA, Cho ES. 2016; Requirement of Smad4-mediated signaling in odontoblast differentiation and dentin matrix formation. Anat Cell Biol. 49:199–205. DOI: 10.5115/acb.2016.49.3.199. PMID: 27722013. PMCID: PMC5052229.
Article38. Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. 2006; Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 235:248–59. DOI: 10.1016/j.canlet.2005.04.033. PMID: 15975709.
Article39. Rugină D, Sconţa Z, Leopold L, Pintea A, Bunea A, Socaciu C. 2012; Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J Med Food. 15:700–6. DOI: 10.1089/jmf.2011.0246. PMID: 22846076. PMCID: PMC3407391.
Article40. Xu M, Bower KA, Wang S, Frank JA, Chen G, Ding M, Wang S, Shi X, Ke Z, Luo J. 2010; Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol Cancer. 9:285. DOI: 10.1186/1476-4598-9-285. PMID: 21034468. PMCID: PMC2984473.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyanidin-3-glucoside Inhibits ATP-induced Intracellular Free Ca2+ Concentration, ROS Formation and Mitochondrial Depolarization in PC12 Cells
- Cyanidin-3-glucoside inhibits amyloid β₂₅₋₃₅-induced neuronal cell death in cultured rat hippocampal neurons
- An Exploration of the Oryza sativa L. Cyanidin-3-glucoside on the Cognitive Function in Older Adults with Subjective Memory Impairment
- Effect of Indole-3-Carbinol on Inhibition of MMP Activity via MAPK Signaling Pathway in Human Prostate Cancer Cell Line, PC3 Cells
- Effect of Cyanidin on Cell Motility and Invasion in MDA-MB-231 Human Breast Cancer Cells