Korean J Gastroenterol.
2020 Nov;76(5):256-260. 10.4166/kjg.2020.111.
Seroreversion and Acute Decompensation in Chronic Hepatitis B after Discontinuation of Oral Nucleotide Analog in the Patients Achieving HBsAg Loss
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- KMID: 2508876
- DOI: http://doi.org/10.4166/kjg.2020.111
Abstract
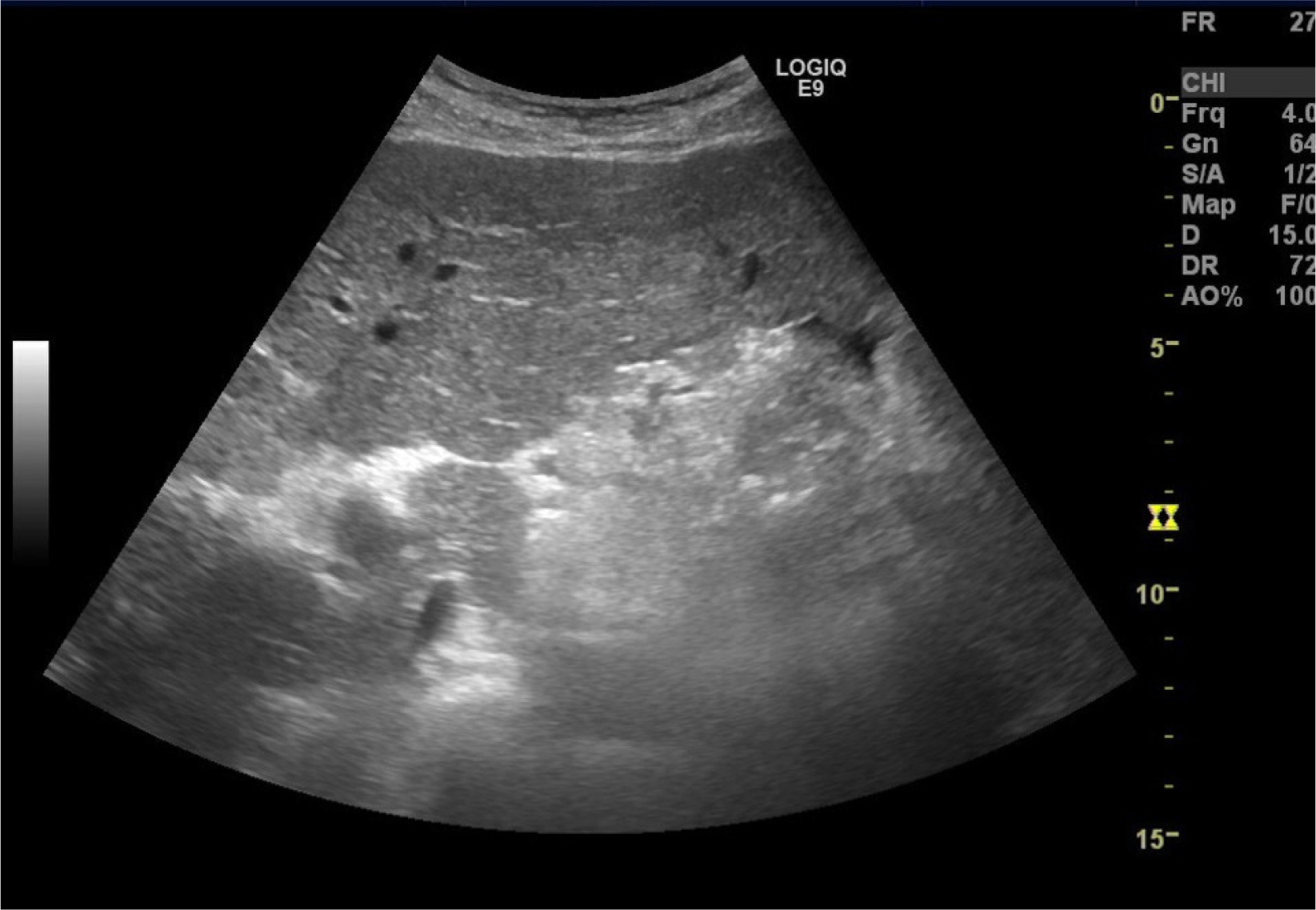
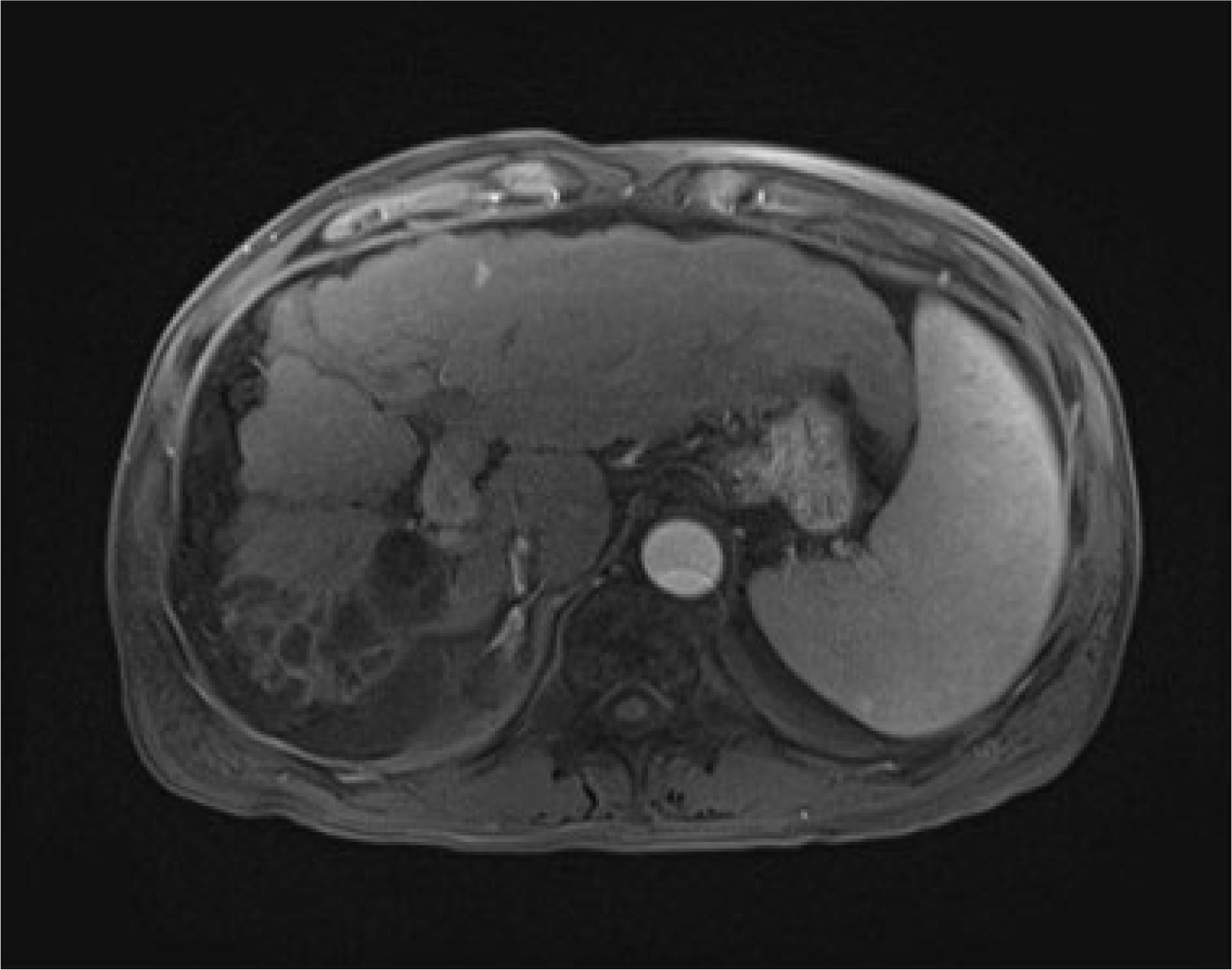
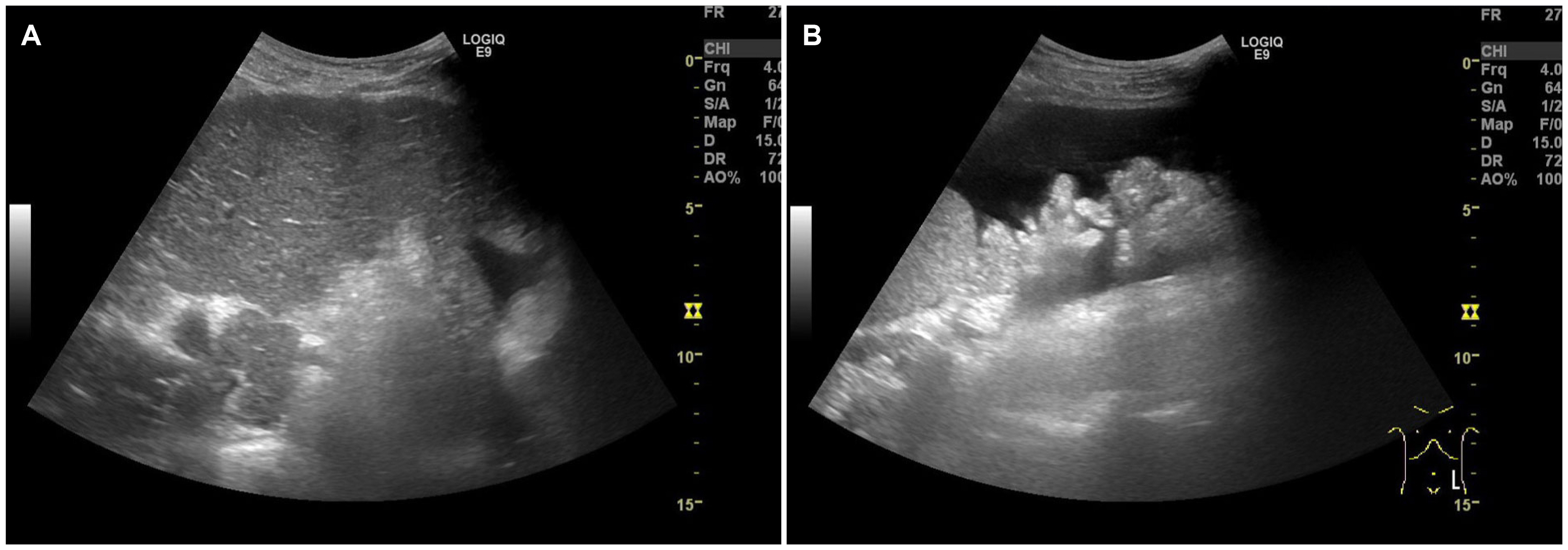
- Although rare patients with chronic hepatitis B can achieve HBsAg loss on oral nucleos(t)ide analog (NA), the optimal timing of stopping oral NAs safely has been considered when HBsAg and HBV DNA are negative in the serum because HBsAg loss induced by nucleos(t)ide analogs (NAs) appears to be durable if immunosuppressive therapy or chemotherapy are not done. On the other hand, the author experienced a case of HBsAg seroreversion and acute decompensation after the discontinuation of NA in a patient with HBsAg loss. This rare case highlights the need for the close monitoring of patients who achieved HBsAg loss and stopped NA.
Figure
Reference
-
1. Calvaruso V, Craxì A. 2011; Fibrosis in chronic viral hepatitis. Best Pract Res Clin Gastroenterol. 25:219–230. DOI: 10.1016/j.bpg.2011.02.012. PMID: 21497740.
Article2. Cornberg M, Lok AS, Terrault NA, Zoulim F. 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. 2020; Guidance for design and endpoints of clinical trials in chronic hepatitis B - report from the 2019 EASL-AASLD HBV treatment endpoints conference‡. J Hepatol. 72:539–557. DOI: 10.1016/j.jhep.2019.11.003. PMID: 31730789.3. European Association for the Study of the Liver. European Association for the Study of the Liver. 2017; EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatology. 67:370–398. DOI: 10.3760/cma.j.issn.1007-3418.2017.06.005. PMID: 28763857.4. Terrault NA, Lok ASF, McMahon BJ, et al. 2018; Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599. DOI: 10.1002/hep.29800. PMID: 29405329. PMCID: PMC5975958.
Article5. European Association For The Study Of The Liver. 2012; EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 57:167–185. DOI: 10.1016/j.jhep.2012.02.010. PMID: 22436845.6. Kim GA, Lim YS, An J, et al. 2014; HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 63:1325–1332. DOI: 10.1136/gutjnl-2013-305517. PMID: 24162593.
Article7. Cornberg M, Höner Zu Siederdissen C. 2014; HBsAg seroclearance with NUCs: rare but important. Gut. 63:1208–1209. DOI: 10.1136/gutjnl-2013-306221. PMID: 24398879.
Article8. Zoulim F. 2005; New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol. 42:302–308. DOI: 10.1016/j.jhep.2004.12.015. PMID: 15710212.
Article9. Martinot-Peignoux M, Lapalus M, Asselah T, Marcellin P. 2014; HBsAg quantification: useful for monitoring natural history and treatment outcome. Liver Int. 34 Suppl 1:97–107. DOI: 10.1111/liv.12403. PMID: 24373085.
Article10. Cornberg M, Wong VW, Locarnini S, Brunetto M, Janssen HLA, Chan HL. 2017; The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 66:398–411. DOI: 10.1016/j.jhep.2016.08.009. PMID: 27575311.
Article11. Yip TC, Wong GL, Wong VW, et al. 2018; Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol. 68:63–72. DOI: 10.1016/j.jhep.2017.09.018. PMID: 28989093.
Article12. Terrault NA, Bzowej NH, Chang KM, et al. 2016; AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 63:261–283. DOI: 10.1002/hep.28156. PMID: 26566064. PMCID: PMC5987259.
Article13. Hu HH, Liu J, Chang CL, et al. 2019; Level of hepatitis B (HB) core antibody associates with seroclearance of HBV DNA and HB surface antigen in HB e antigen-seronegative patients. Clin Gastroenterol Hepatol. 17:172–181.e1. DOI: 10.1016/j.cgh.2018.04.064. PMID: 29753083.
Article14. Testoni B, Lebossé F, Scholtes C, et al. 2019; Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 70:615–625. DOI: 10.1016/j.jhep.2018.11.030. PMID: 30529504.
Article15. Liu F, Wang XW, Chen L, Hu P, Ren H, Hu HD. 2016; Systematic review with meta-analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther. 43:1253–1261. DOI: 10.1111/apt.13634. PMID: 27117732.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mutations at the Gene Encoding the 'a' Determinant of HBsAg in Chronic Hepatitis B Patients with Concurrent HBsAg and Anti-HBs Positivity
- Viral Serologic Markers in Patients with Acute and Chronic Liver Diseases
- Production of IFN-gamma by HBsAg - reactive T cells correlates with viral clearance in HBV infection
- Unresolved Problems on Current Antiviral Treatment for Chronic Hepatitis B in Korea
- Prevalence of Australia Antigen and Antibody in Pediatric Age Group