Ann Hepatobiliary Pancreat Surg.
2020 Nov;24(4):396-414. 10.14701/ahbps.2020.24.4.396.
Perioperative immunonutrition in hepatectomy: A systematic review and meta-analysis
- Affiliations
-
- 1Department of Hepatopancreaticobiliary Surgery, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
- 2University of Edinburgh, Edinburgh, UK
- 3Royal College of Surgeons of Edinburgh, Edinburgh, UK
- KMID: 2508848
- DOI: http://doi.org/10.14701/ahbps.2020.24.4.396
Abstract
- Backgrounds/Aims
The role of immunonutrition (IMN) after liver resections or hepatectomies remains unclear and controversial. We undertook a systematic review to evaluate the effects of IMN on clinical outcomes of patients undergoing hepatectomy.
Methods
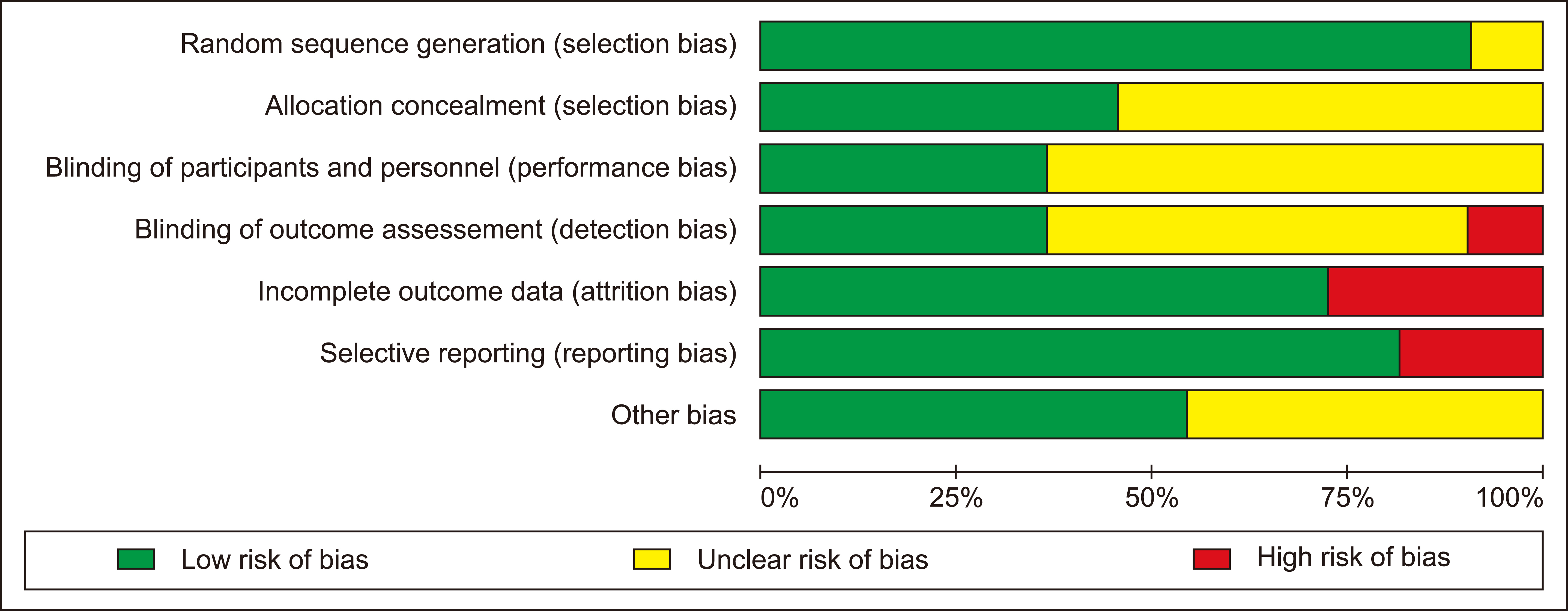
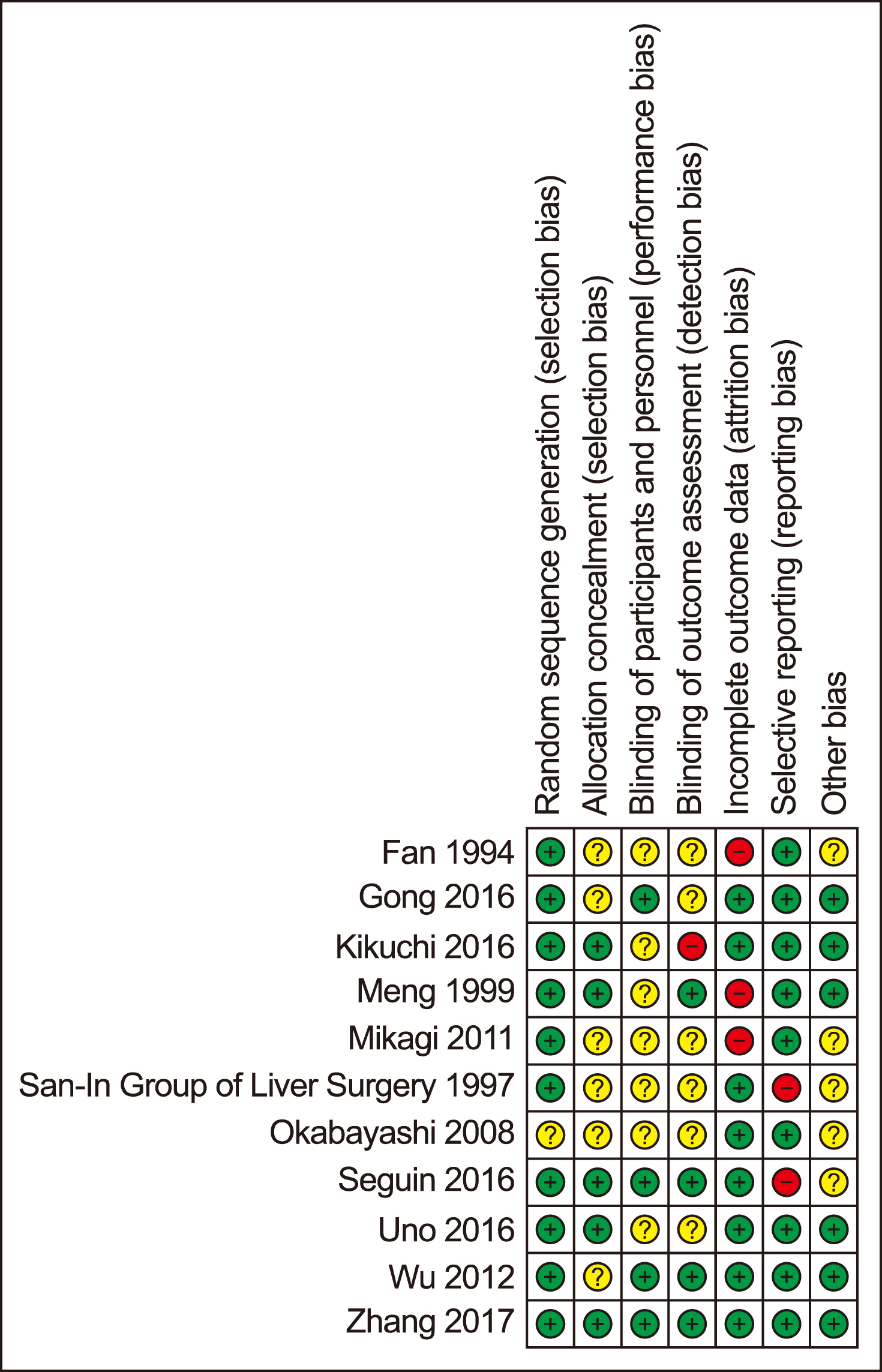
Main electronic databases were searched for randomised trials reported clinical outcomes or effects of IMN. The systematic review was conducted in accordance with the PRISMA guideline and meta-analysis was analysed using fixed or random-effects models.
Results
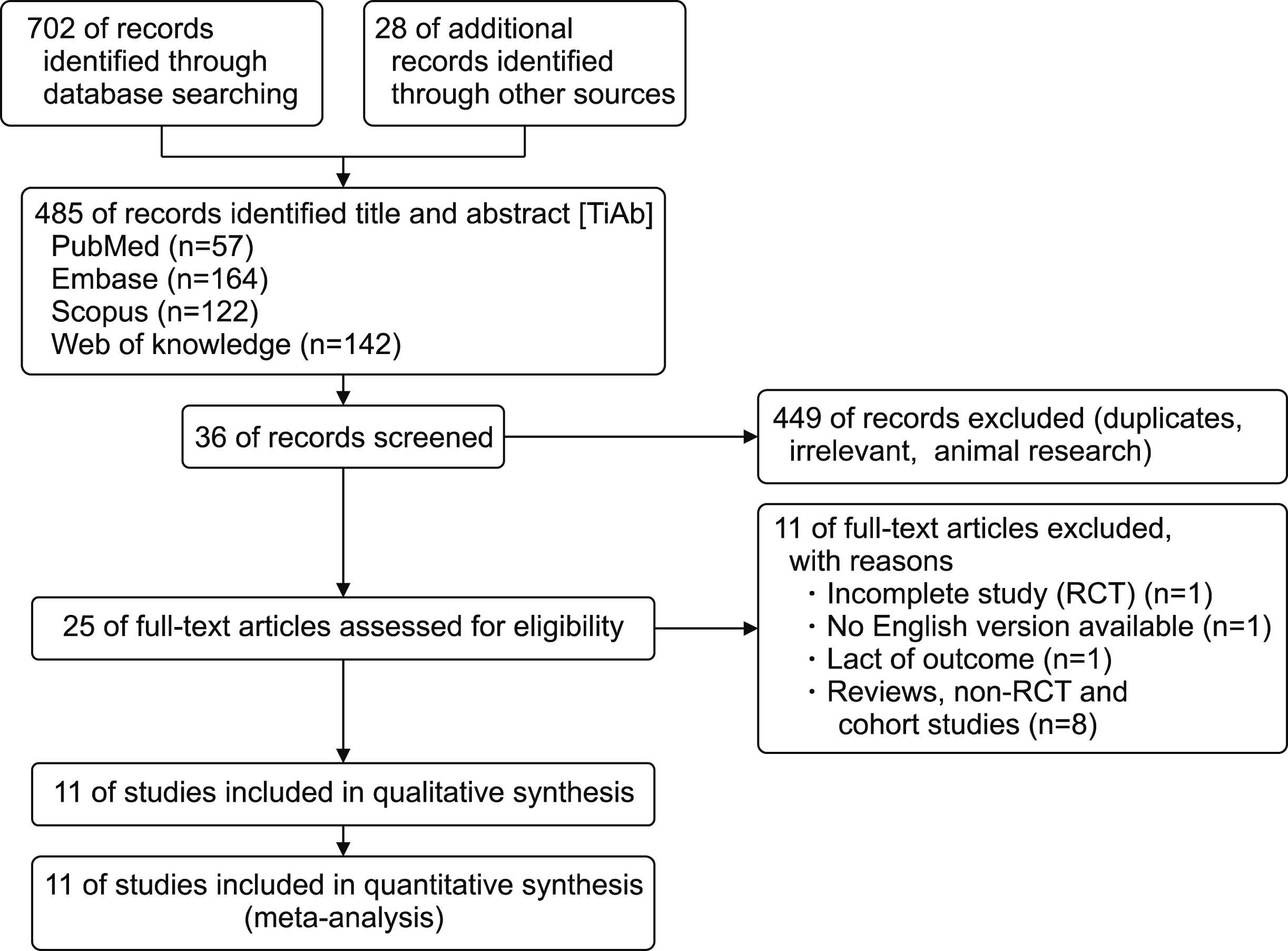
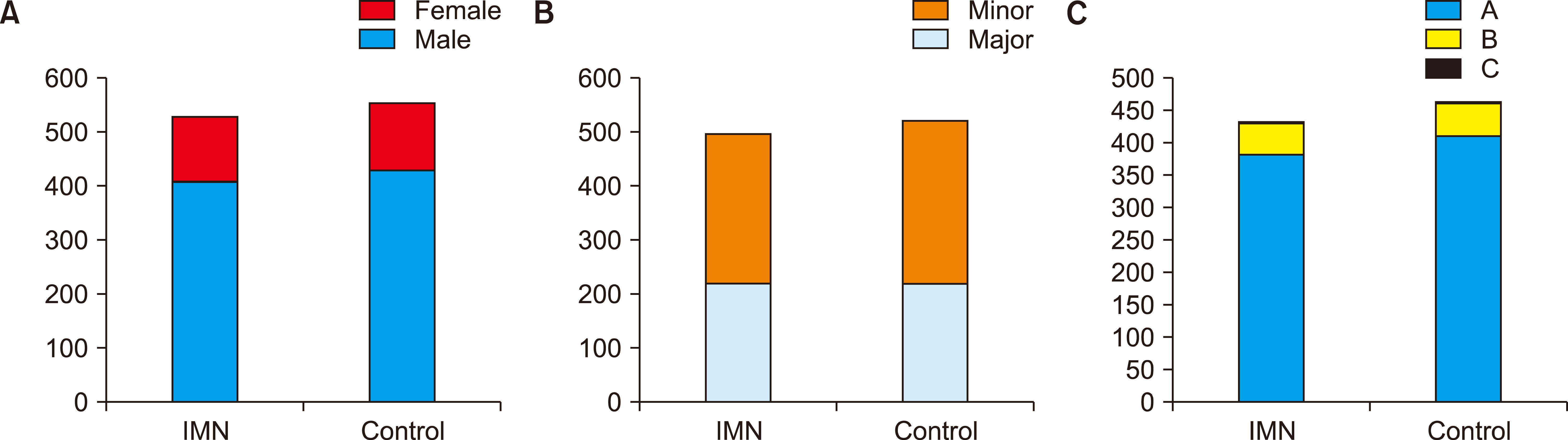
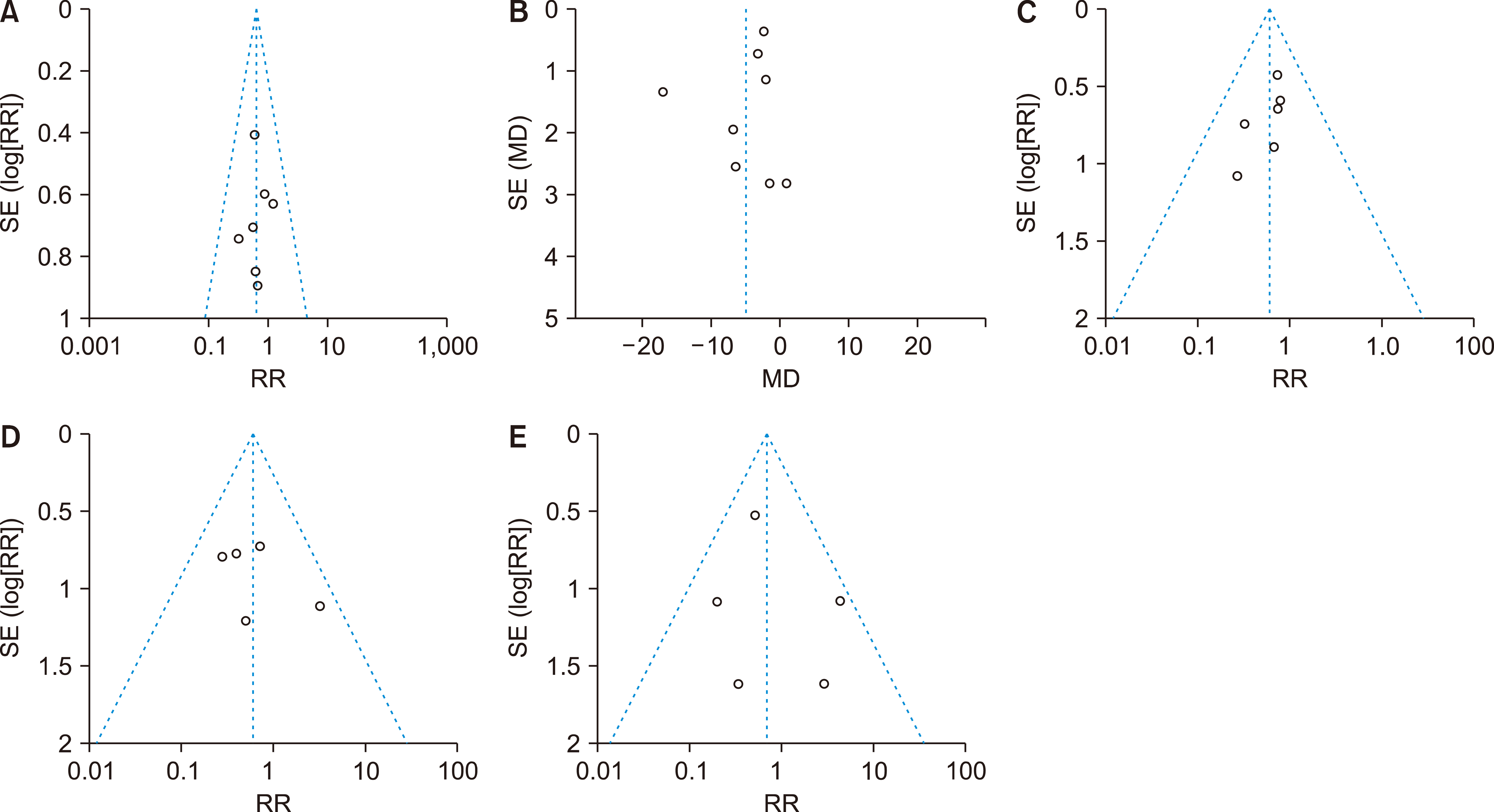
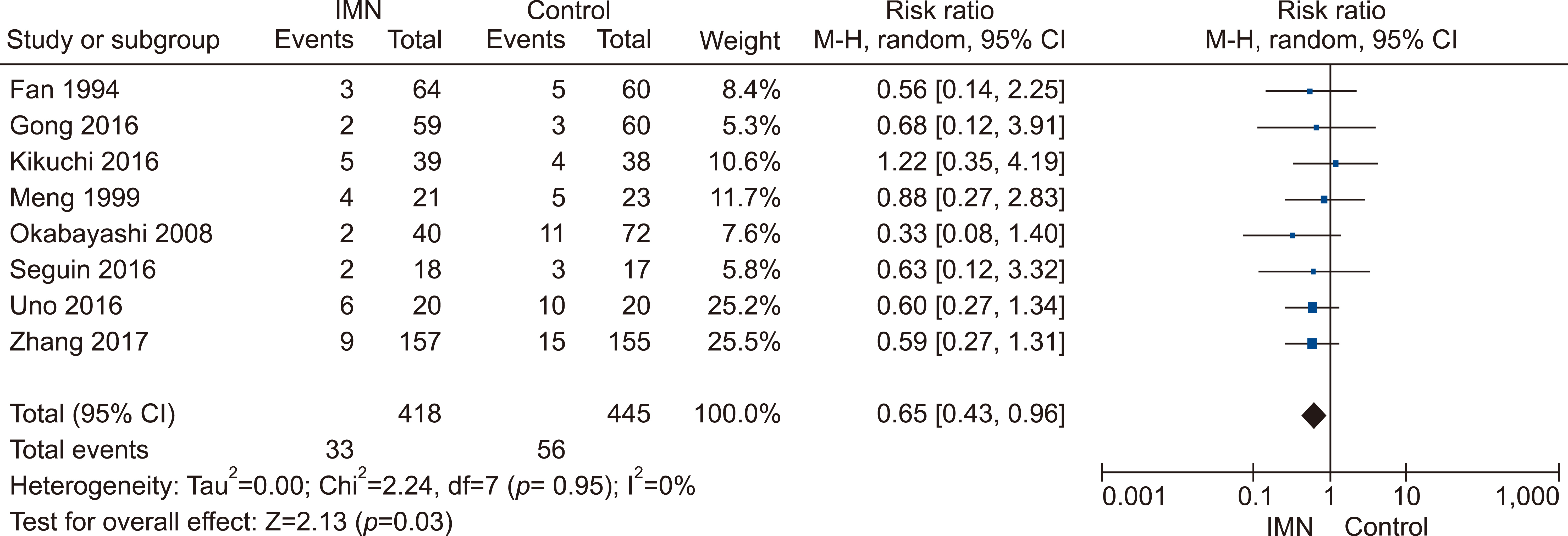
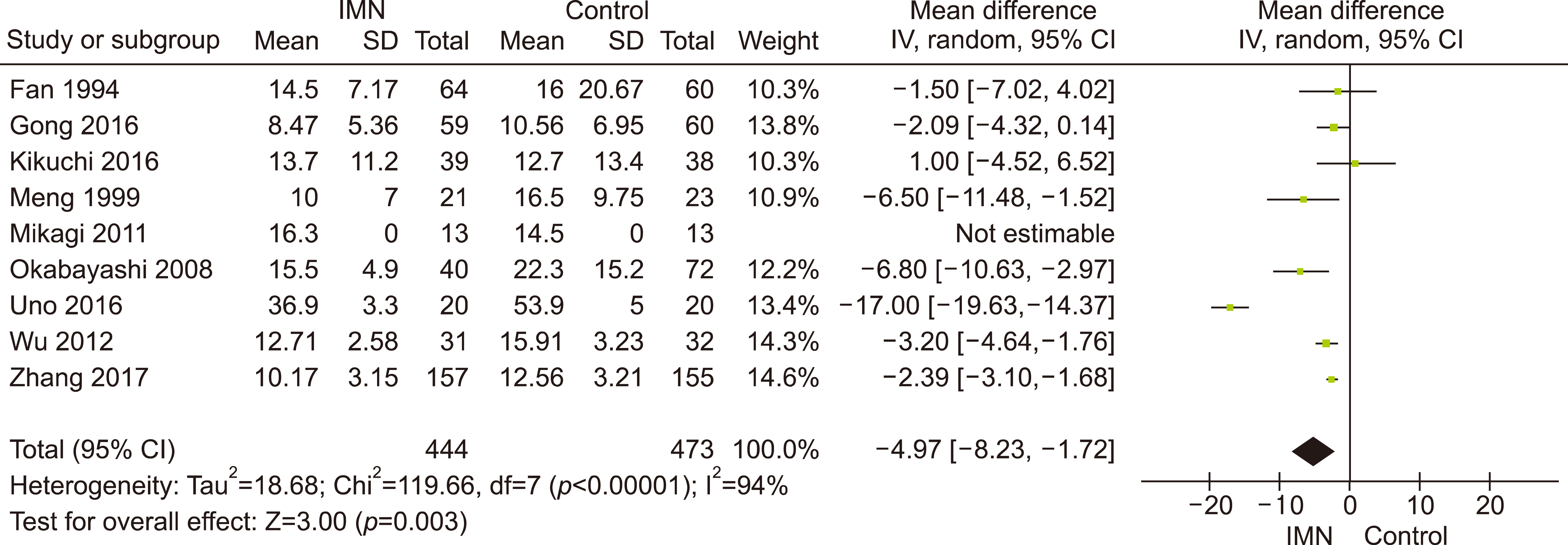
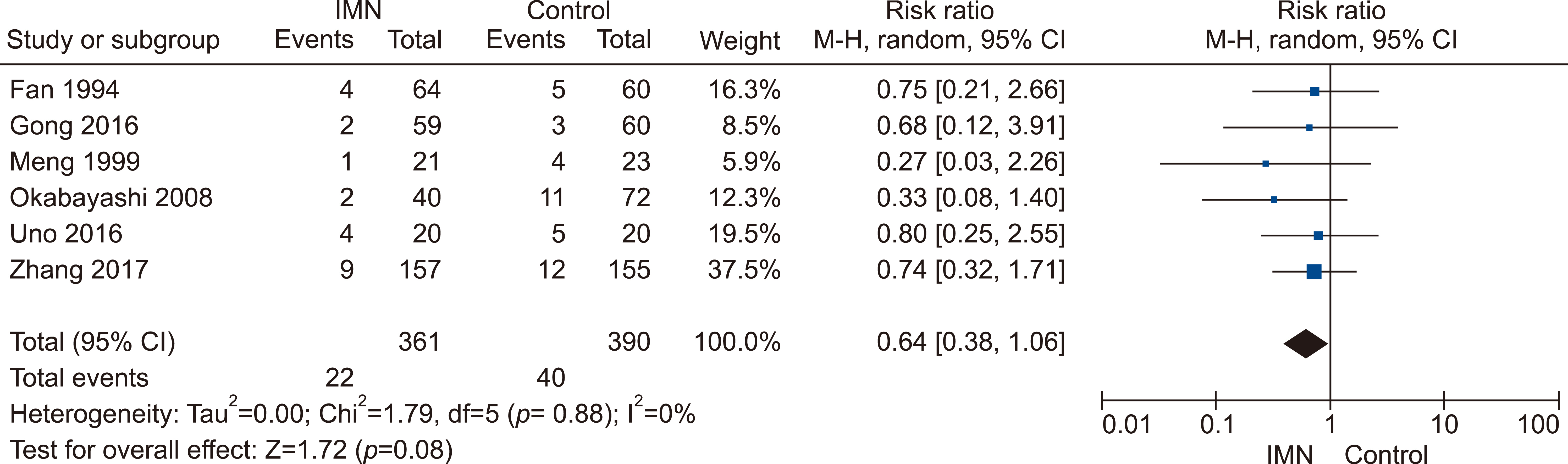
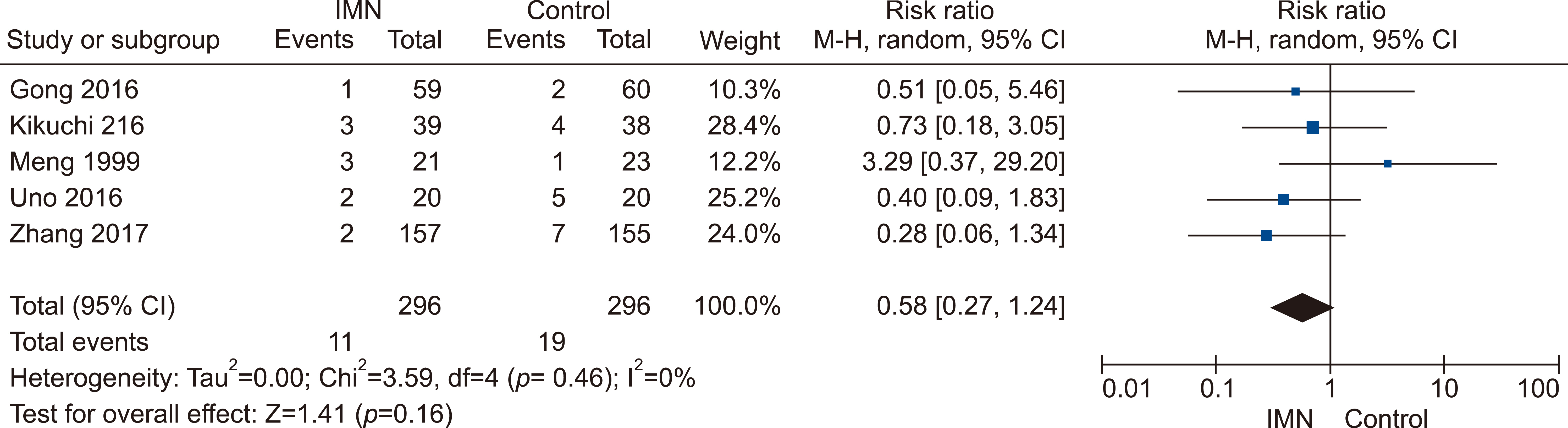
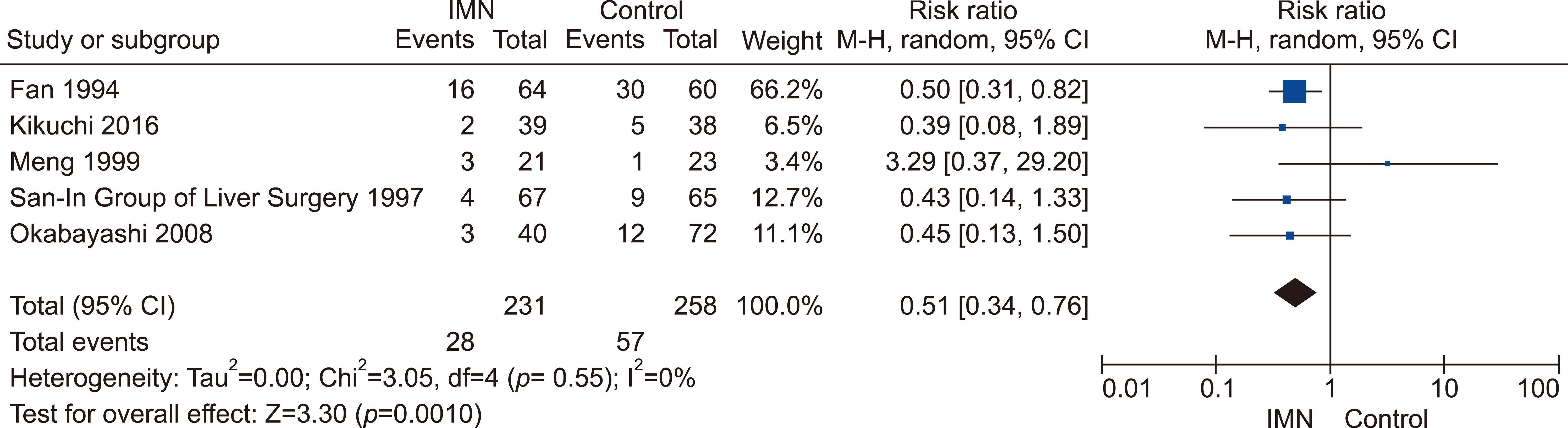
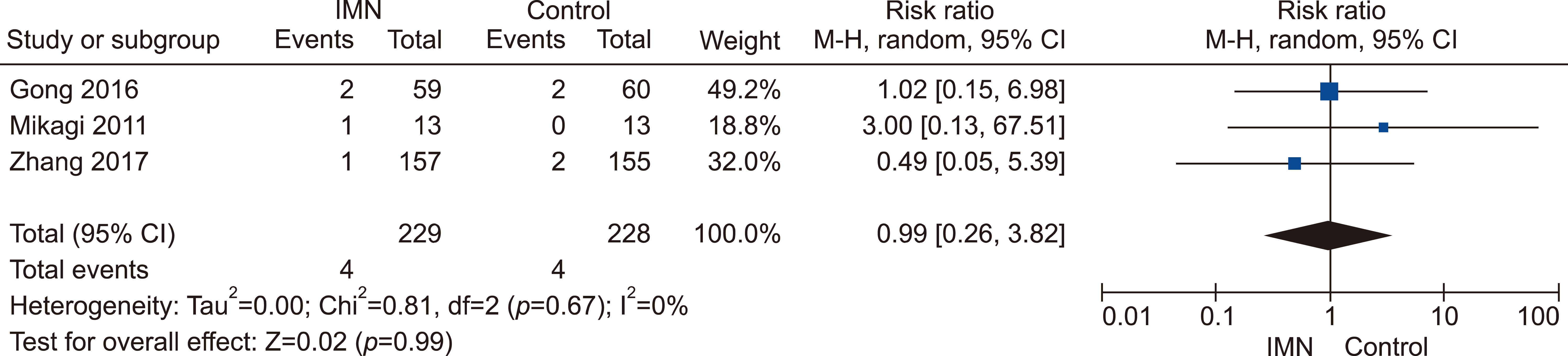
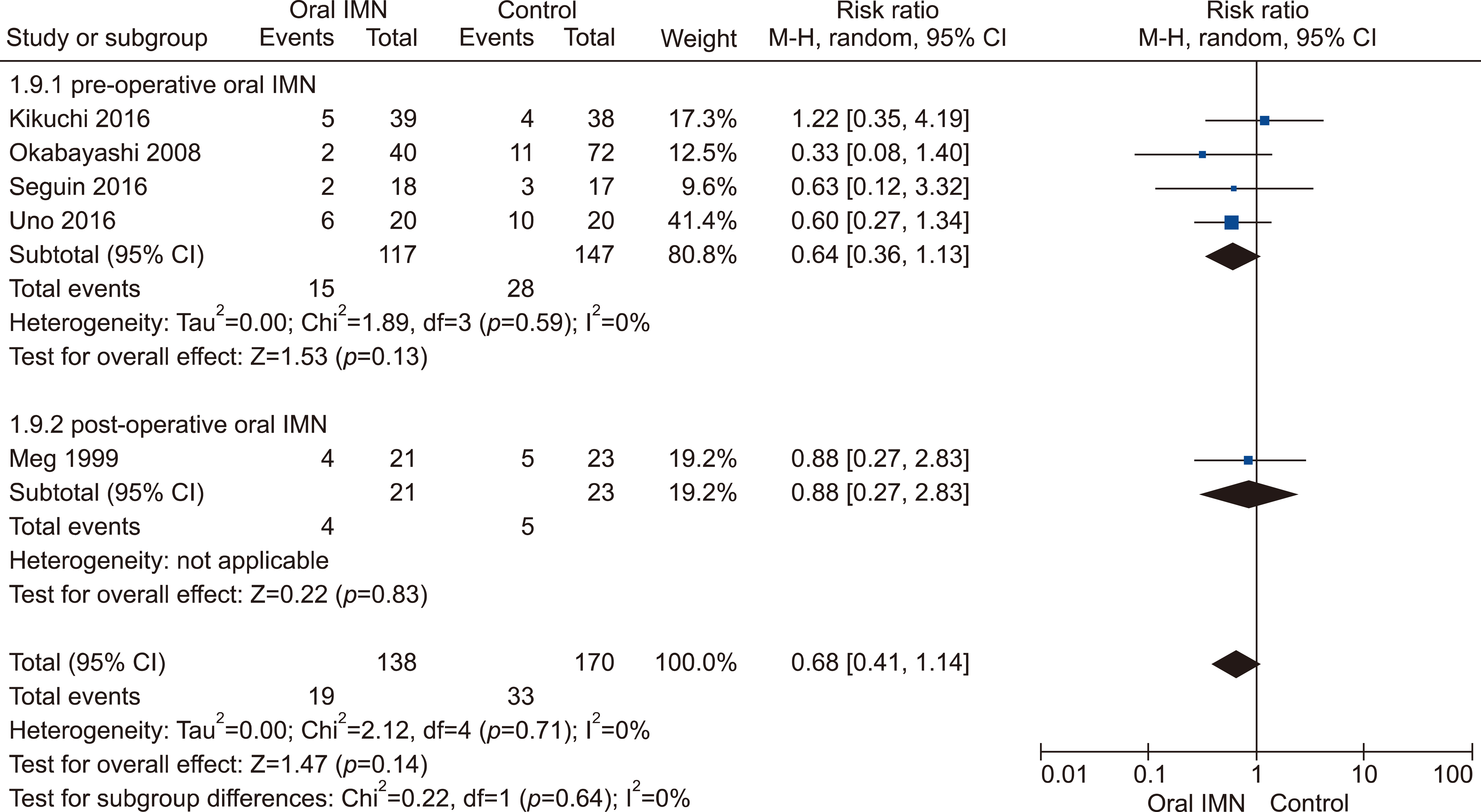
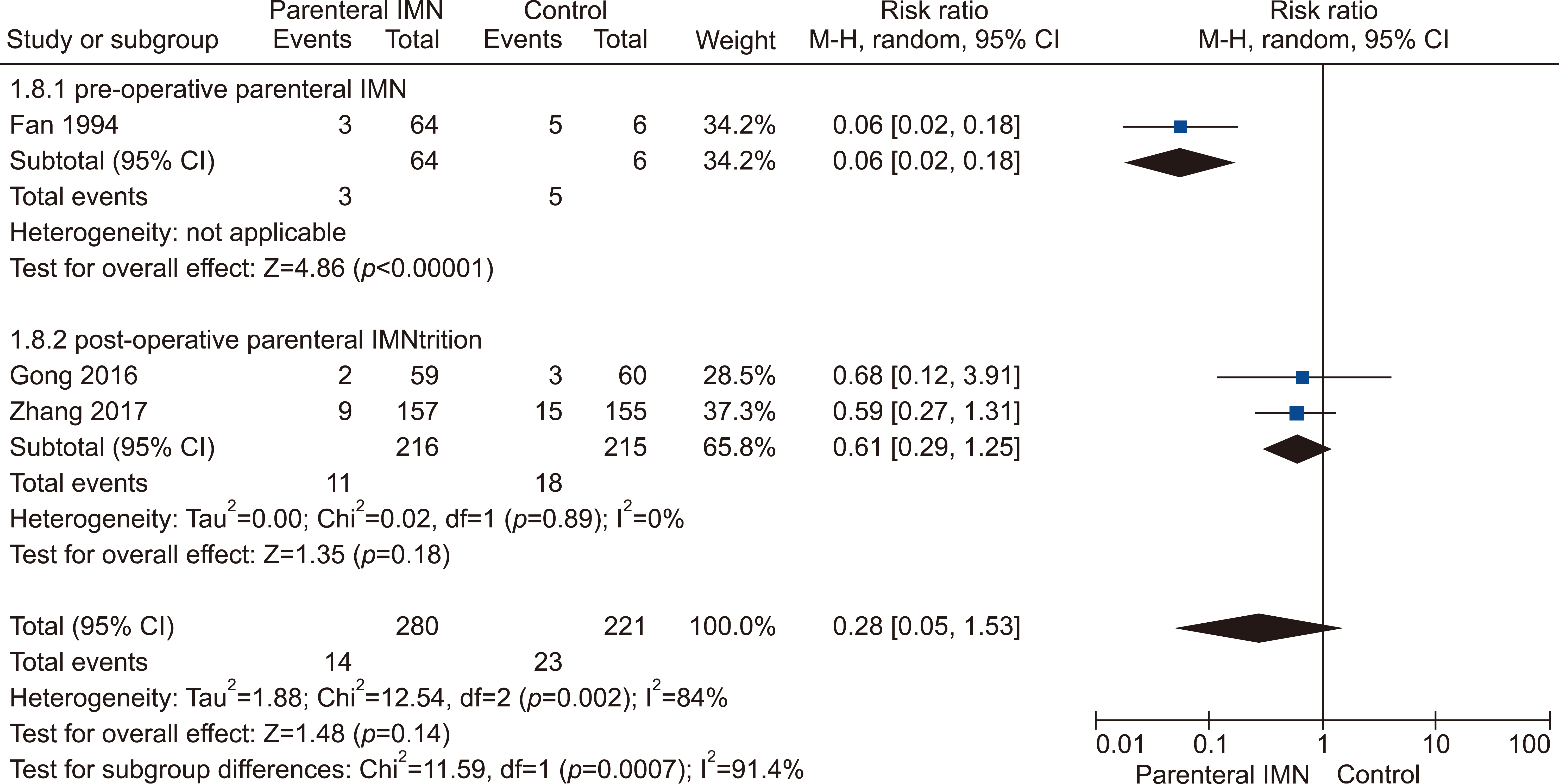
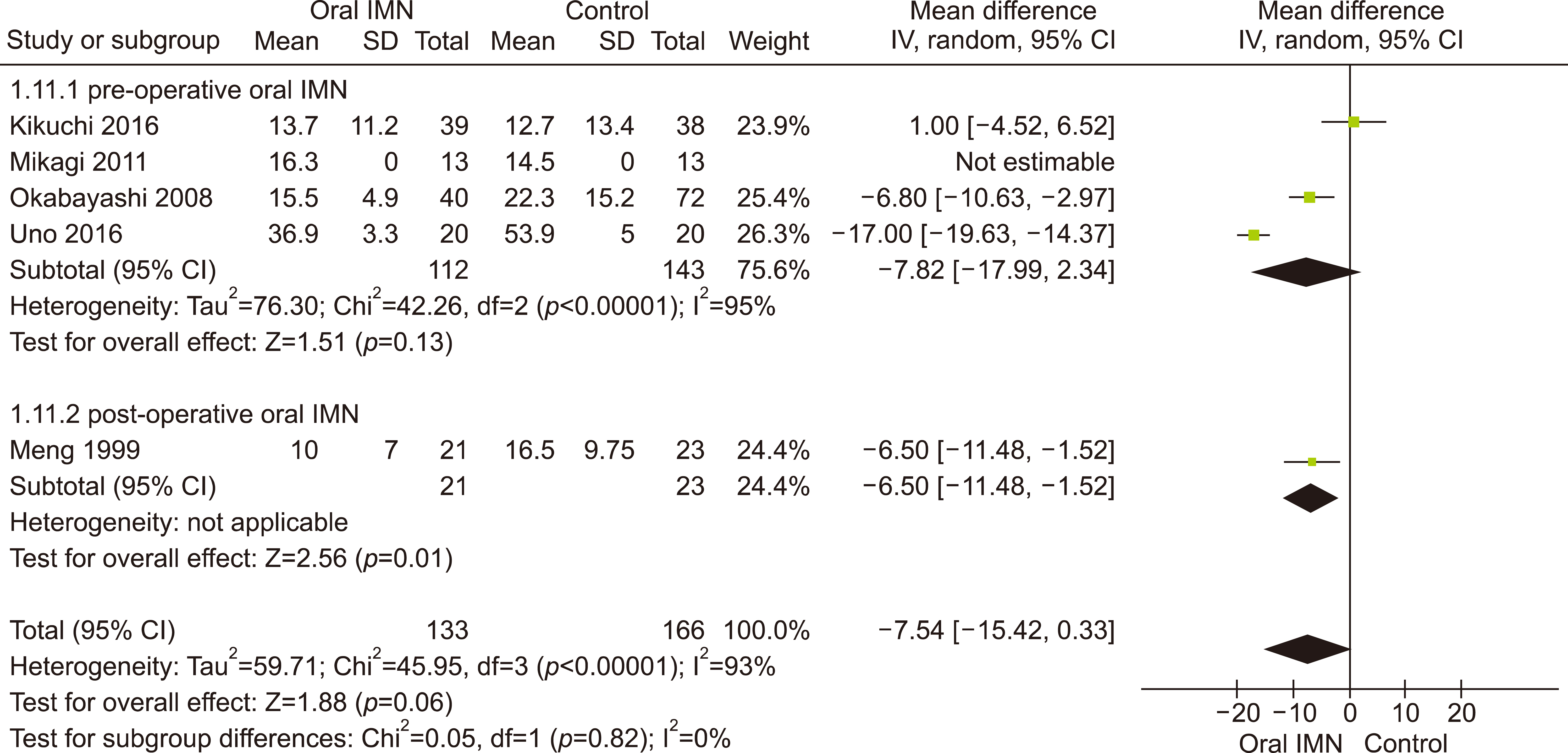
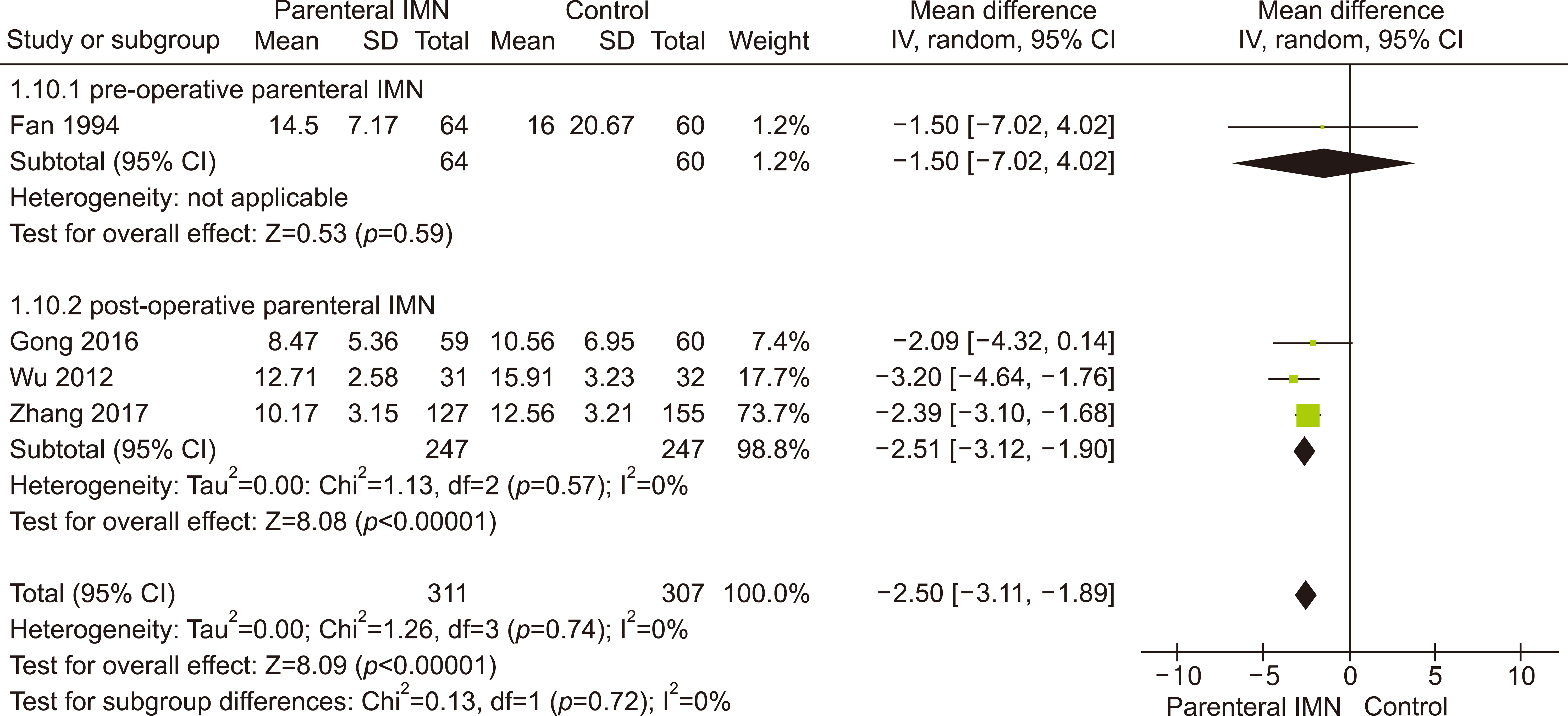
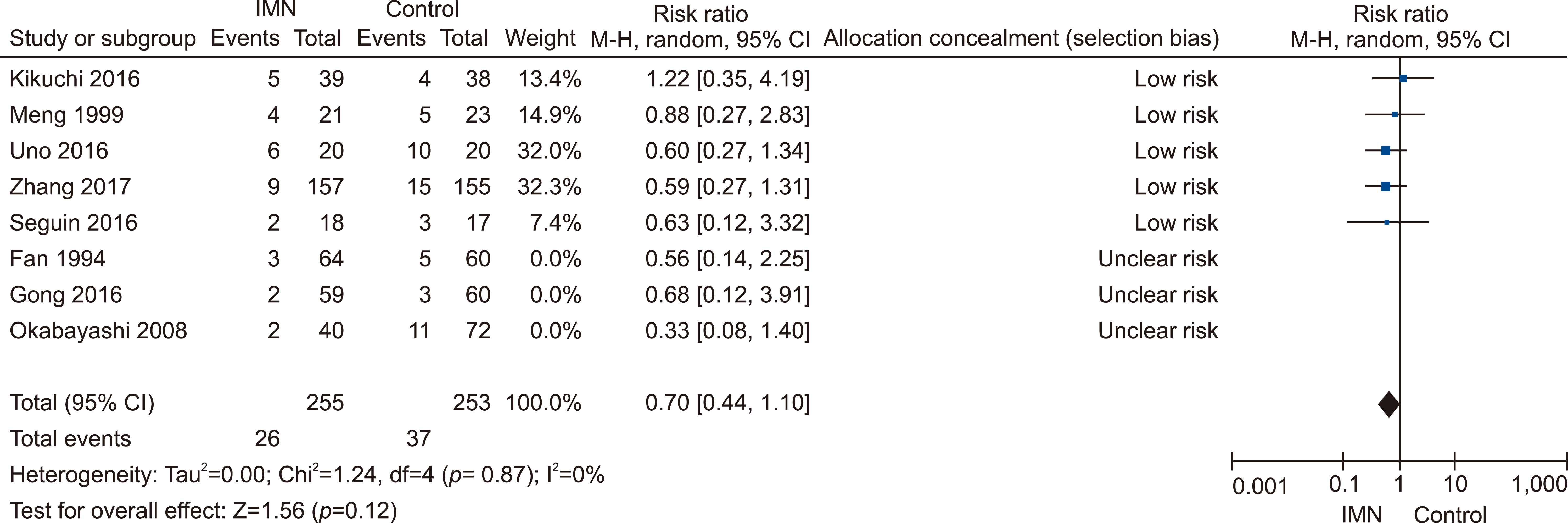
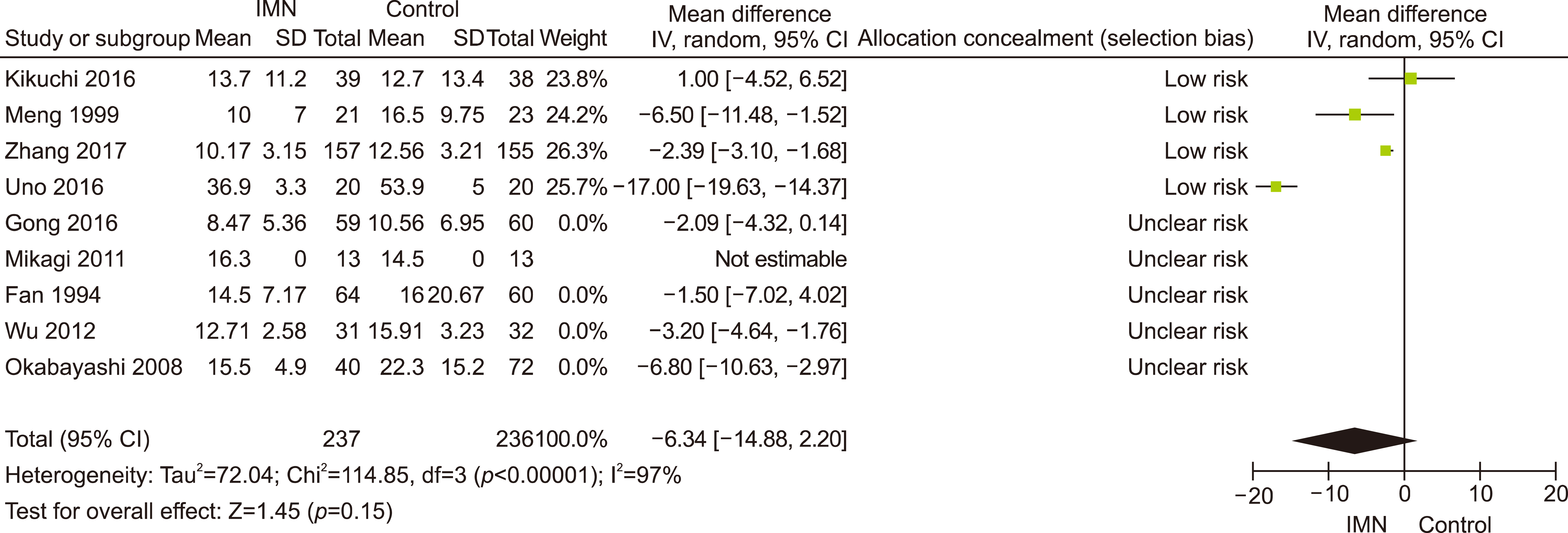
Eleven RCTs were identified. A total of 1084 patients (529 IMN and 555 Control) were included in the final pooled analysis. Of these patients, 43% (440/1016) underwent major hepatectomies and the majority are for hepatocellular carcinoma (90%, 956/1055) with Child-Pugh A disease (89%, 793/894). IMN significantly reduced post-operative wound infection (risk ratio (RR) 0.65, 95% confidence interval (CI) 0.43 to 0.96; p=0.03). IMN also had a shorter hospital stay (MD −4.97 days, 95% CI −8.23 to −1.72; p=0.003). There was no statistically significant in other post-operative morbidities and mortality.
Conclusions
Wound infection rate was not significantly different between oral and parenteral IMN group. The length of hospital stay was significantly lower in parenteral IMN group than in oral IMN group. The mortality rates were not affected. Immunonutrition should be recommended routinely as part of the nutritional support in the Enhanced Recovery after Surgery (ERAS) protocol for hepatectomy.
Keyword
Figure
Reference
-
1. Kehlet H. 1997; Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 78:606–617. DOI: 10.1093/bja/78.5.606. PMID: 9175983.
Article2. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. 2017; ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 36:623–650. DOI: 10.1016/j.clnu.2017.02.013. PMID: 28385477.
Article3. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. 2009; ESPEN guidelines on parenteral nutrition: surgery. Clin Nutr. 28:378–386. DOI: 10.1016/j.clnu.2009.04.002. PMID: 19464088.
Article4. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. 2016; Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 40:159–211. DOI: 10.1177/0148607115621863. PMID: 26773077.5. Brennan MF, Pisters PW, Posner M, Quesada O, Shike M. 1994; A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg. 220:436–441. discussion 441–444. DOI: 10.1097/00000658-199410000-00003. PMID: 7944656. PMCID: PMC1234412.
Article6. Evans DC, Martindale RG, Kiraly LN, Jones CM. 2014; Nutrition optimization prior to surgery. Nutr Clin Pract. 29:10–21. DOI: 10.1177/0884533613517006. PMID: 24347529.
Article7. Lin E, Goncalves JA, Lowry SF. 1998; Efficacy of nutritional pharmacology in surgical patients. Curr Opin Clin Nutr Metab Care. 1:41–50. DOI: 10.1097/00075197-199801000-00008. PMID: 10565329.
Article8. Marik PE, Zaloga GP. 2010; Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr. 34:378–386. DOI: 10.1177/0148607110362692. PMID: 20631383.9. Hegazi RA, Hustead DS, Evans DC. 2014; Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg. 219:1078–1087. DOI: 10.1016/j.jamcollsurg.2014.06.016. PMID: 25260681.
Article10. Probst P, Ohmann S, Klaiber U, Hüttner FJ, Billeter AT, Ulrich A, et al. 2017; Meta-analysis of immunonutrition in major abdominal surgery. Br J Surg. 104:1594–1608. DOI: 10.1002/bjs.10659. PMID: 28940219.
Article11. Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW, et al. 2011; A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford). 13:494–502. DOI: 10.1111/j.1477-2574.2011.00330.x. PMID: 21689233. PMCID: PMC3133716.
Article12. Fischer JE. 2012. Fischer's mastery of surgery. 6th ed. Wolters Kluwer Health/Lippincott Williams & Wilkins;Philadelphia:13. Lillemoe K, Jarnagin W. 2012. Master techniques in surgery: hepatobiliary and pancreatic surgery. Wolters Kluwer;Philadelphia:14. Garden OJ, Parks RW. 2014. Hepatobiliary and pancreatic surgery. 5th ed. Saunders Elsevier;Edinburgh:15. Cerantola Y, Hübner M, Grass F, Demartines N, Schäfer M. 2011; Immunonutrition in gastrointestinal surgery. Br J Surg. 98:37–48. DOI: 10.1002/bjs.7273. PMID: 20931620.
Article16. Drover JW, Dhaliwal R, Weitzel L, Wischmeyer PE, Ochoa JB, Heyland DK. 2011; Perioperative use of arginine-supplemented diets: a systematic review of the evidence. J Am Coll Surg. 212:385–399. 3990. DOI: 10.1016/j.jamcollsurg.2010.10.016. PMID: 21247782.
Article17. Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. 2012; A meta- analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg. 255:1060–1068. DOI: 10.1097/SLA.0b013e318252edf8. PMID: 22549749.18. Moher D, Liberati A, Tetzlaff J, Altman DG. 2009; Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. DOI: 10.1371/journal.pmed.1000097. PMID: 19621072. PMCID: PMC2707599.
Article19. Hozo SP, Djulbegovic B, Hozo I. 2005; Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 5:13. DOI: 10.1186/1471-2288-5-13. PMID: 15840177. PMCID: PMC1097734.
Article20. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. 1996; Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 17:1–12. DOI: 10.1016/0197-2456(95)00134-4. PMID: 8721797.
Article21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. 2011; The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 343:d5928. DOI: 10.1136/bmj.d5928. PMID: 22008217. PMCID: PMC3196245.
Article22. Egger M, Smith GD, Altman DG. 2001. Systematic reviews in health care: meta-analysis in context. 2nd ed. BMJ;London: DOI: 10.1002/9780470693926.23. Mantel N, Haenszel W. 1959; Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 22:719–748. PMID: 13655060.24. DerSimonian R, Laird N. 1986; Meta-analysis in clinical trials. Control Clin Trials. 7:177–188. DOI: 10.1016/0197-2456(86)90046-2. PMID: 3802833.
Article25. Higgins JP, Thompson SG. 2002; Quantifying heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. DOI: 10.1002/sim.1186. PMID: 12111919.
Article26. Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. 1994; Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 331:1547–1552. DOI: 10.1056/NEJM199412083312303. PMID: 7969324.
Article27. San‐In Group of Liver Surgery. 1997; Long-term oral administration of branched chain amino acids after curative resection of hepatocellular carcinoma: a prospective randomized trial. Br J Surg. 84:1525–1531. DOI: 10.1111/j.1365-2168.1997.02810.x. PMID: 9393270.28. Meng WC, Leung KL, Ho RL, Leung TW, Lau WY. 1999; Prospective randomized control study on the effect of branched-chain amino acids in patients with liver resection for hepatocellular carcinoma. Aust N Z J Surg. 69:811–815. DOI: 10.1046/j.1440-1622.1999.01701.x. PMID: 10553972.
Article29. Okabayashi T, Nishimori I, Sugimoto T, Maeda H, Dabanaka K, Onishi S, et al. 2008; Effects of branched-chain amino acids-enriched nutrient support for patients undergoing liver resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 23:1869–1873. DOI: 10.1111/j.1440-1746.2008.05504.x. PMID: 18717761.
Article30. Mikagi K, Kawahara R, Kinoshita H, Aoyagi S. 2011; Effect of preoperative immunonutrition in patients undergoing hepatectomy; a randomized controlled trial. Kurume Med J. 58:1–8. DOI: 10.2739/kurumemedj.58.1. PMID: 22027191.
Article31. Wu Z, Qin J, Pu L. 2012; Omega-3 fatty acid improves the clinical outcome of hepatectomized patients with hepatitis B virus (HBV)- associated hepatocellular carcinoma. J Biomed Res. 26:395–399. DOI: 10.7555/JBR.26.20120058. PMID: 23554777. PMCID: PMC3597052.32. Gong Y, Liu Z, Liao Y, Mai C, Chen T, Tang H, et al. 2016; Effectiveness of ɷ-3 polyunsaturated fatty acids based lipid emulsions for treatment of patients after hepatectomy: a prospective clinical trial. Nutrients. 8:357. DOI: 10.3390/nu8060357. PMID: 27322311. PMCID: PMC4924198.
Article33. Seguin P, Locher C, Boudjema K, Hamon C, Mouchel C, Malledant Y, et al. 2016; Effect of a perioperative nutritional supplementation with Oral ImpactⓇ in patients undergoing hepatic surgery for liver cancer: a prospective, placebo-controlled, randomized, double-blind study. Nutr Cancer. 68:464–472. DOI: 10.1080/01635581.2016.1153670. PMID: 27007018.
Article34. Uno H, Furukawa K, Suzuki D, Shimizu H, Ohtsuka M, Kato A, et al. 2016; Immunonutrition suppresses acute inflammatory responses through modulation of resolvin E1 in patients undergoing major hepatobiliary resection. Surgery. 160:228–236. DOI: 10.1016/j.surg.2016.01.019. PMID: 26965712.
Article35. Kikuchi Y, Hiroshima Y, Matsuo K, Kawaguchi D, Murakami T, Yabushita Y, et al. 2016; A randomized clinical trial of preoperative administration of branched-chain amino acids to prevent postoperative ascites in patients with liver resection for hepatocellular carcinoma. Ann Surg Oncol. 23:3727–3735. DOI: 10.1245/s10434-016-5348-3. PMID: 27338747.
Article36. Zhang B, Wei G, Li R, Wang Y, Yu J, Wang R, et al. 2017; n-3 fatty acid-based parenteral nutrition improves postoperative recovery for cirrhotic patients with liver cancer: a randomized controlled clinical trial. Clin Nutr. 36:1239–1244. DOI: 10.1016/j.clnu.2016.08.002. PMID: 27614675.
Article37. Agarwal A, Borley NR, McLatchie GR. 2017. Oxford handbook of operative surgery. 3rd ed. Oxford University Press;Oxford: DOI: 10.1093/med/9780199608911.001.0001.38. Cuschieri A, Steele RJC, Moossa AR. 2002. Essential surgical practice: higher surgical training in general surgery. 4th ed. Arnold;London:39. Garden OJ, Parks RW. 2018. Principles and practice of surgery. 7th ed. Elsevier;Edinburgh:40. Jensen GL, Bistrian B, Roubenoff R, Heimburger DC. 2009; Malnutrition syndromes: a conundrum vs continuum. JPEN J Parenter Enteral Nutr. 33:710–716. DOI: 10.1177/0148607109344724. PMID: 19892905.
Article41. Richard V, Dahiya D, Kaman L, Raj P, Behera A. 2014; Effect of perioperative glutamine administration on C-reactive protein and liver function tests in patients undergoing hepatic resection. Pol Przegl Chir. 86:11–16. DOI: 10.2478/pjs-2014-0003. PMID: 24578449.
Article42. Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, et al. 2006; ESPEN guidelines on Enteral nutrition: surgery including organ transplantation. Clin Nutr. 25:224–244. DOI: 10.1016/j.clnu.2006.01.015. PMID: 16698152.
Article43. Consensus recommendations from the US summitt on immune- enhancing enteral therapy. 2001; JPEN J Parenter Enteral Nutr. 25(2 Suppl):S61–S63. DOI: 10.1177/014860710102500213. PMID: 11288926.44. Braga M, Gianotti L, Vignali A, Schmid A, Nespoli L, Di Carlo V. 2005; Hospital resources consumed for surgical morbidity: effects of preoperative arginine and omega-3 fatty acid supplementation on costs. Nutrition. 21:1078–1086. DOI: 10.1016/j.nut.2005.05.003. PMID: 16308130.45. Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. 2001; The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 134:663–694. DOI: 10.7326/0003-4819-134-8-200104170-00012. PMID: 11304107.
Article