Endocrinol Metab.
2020 Sep;35(3):571-577. 10.3803/EnM.2020.681.
A Phase II Multi-Center, Non-Randomized, Parallel Group, Non-Inferiority Study to Compare the Efficacy of No Radioactive Iodine Remnant Ablation to Remnant Ablation Treatment in Low- to Intermediate-Risk of Papillary Thyroid Cancer: The MOREthyroid Trial Protocol
- KMID: 2508006
- DOI: http://doi.org/10.3803/EnM.2020.681
Abstract
- Background
Radioactive iodine (RAI) remnant ablation is recommended in patients with papillary thyroid cancer (PTC) and extrathyroidal extension or central lymph node metastasis. However, there exists little evidence about the necessity of remnant ablation in PTC patients with low- to intermediate-risk, those have been increasing in recent decades.
Methods
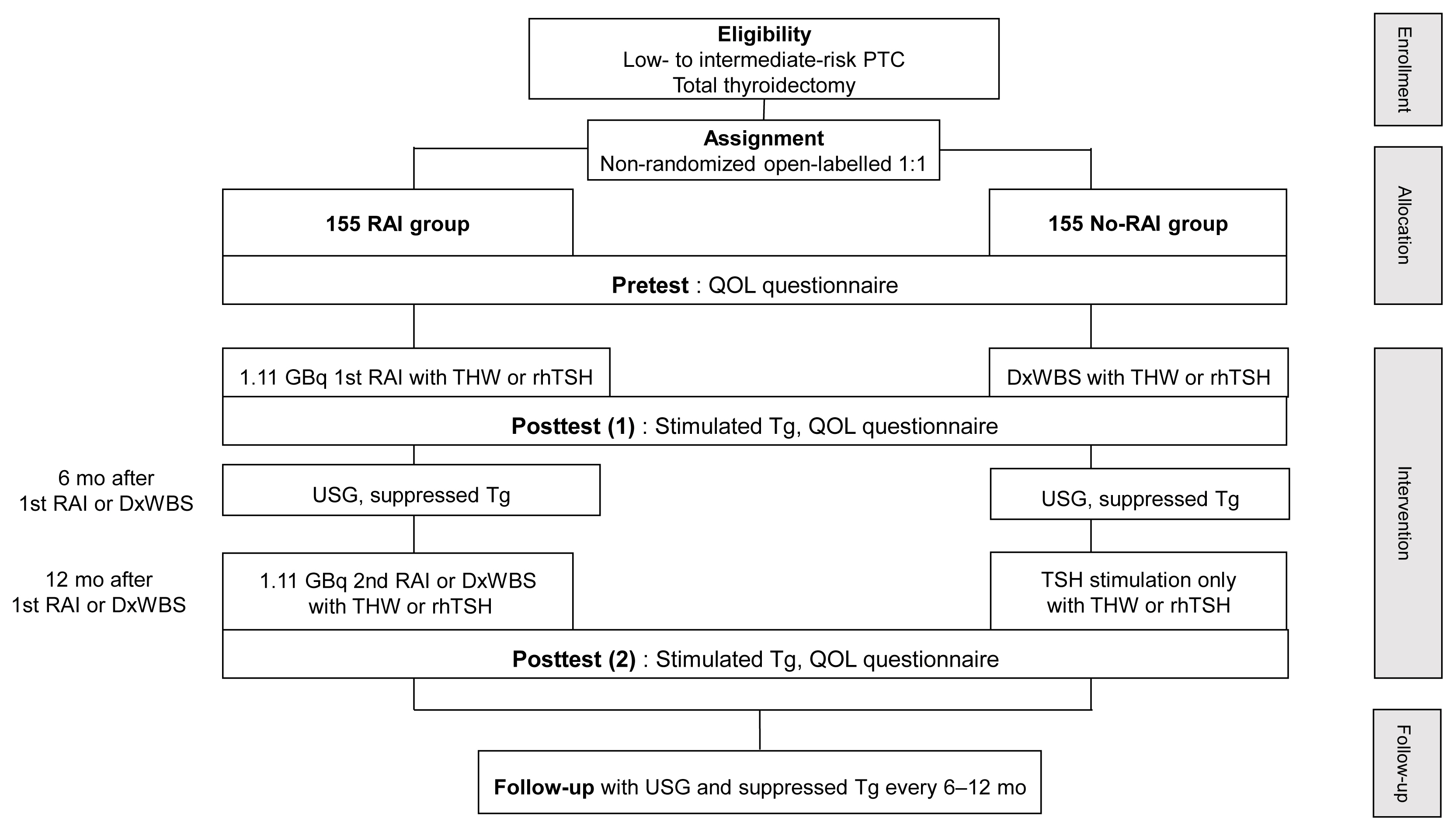
This multicenter, prospective, non-randomized, parallel group clinical trial will enroll 310 eligible patients with low- to intermediate-risk of thyroid cancer. Inclusion criteria are patients who recently underwent total thyroidectomy for PTC with 3 or less tumors of size 1≤ to ≤2 cm with no microscopic extension and N0/x, or size ≤2 cm with microscopic extension and/or N1a (number of lymph node ≤3, size of tumor foci ≤0.2 cm, and lymph node ratio <0.4). Patients choose to undergo RAI ablation (131I, dose 1.1 GBq) or diagnostic whole-body scan (DxWBS) (131I or 123I, dose 0.074 to 0.222 GBq), followed by subsequent measurement of stimulated thyroglobulin (sTg) within 1 year. Survey for quality of life (QOL) will be performed at baseline and at 1 year after follow-up. The total enrollment period is 5 years, and patients will be followed up for 1 year. The primary endpoint is the non-inferiority of surgery alone to surgery with ablation in terms of biochemical remission (BCR) rate (sTg ≤2 ng/mL) without evidence of structural recurrence. The secondary endpoint was the difference of QOL.
Conclusion
This study will evaluate whether surgery alone achieves similar BCR and improved QOL compared to RAI ablation in patients with low- to intermediate-risk PTC within 1 year.
Figure
Reference
-
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article2. Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016; 9:59–126.
Article3. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012; 366:1674–85.
Article4. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012; 366:1663–73.
Article5. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013; 158:200–7.
Article6. Ryu CH, Park B, Ryu J, Ryu YM, Jo SA, Lee YJ, et al. Development and evaluation of a Korean version of a thyroid-specific quality-of-life questionnaire scale in thyroid cancer patients. Cancer Res Treat. 2018; 50:405–15.
Article7. Dow KH, Ferrell BR, Anello C. Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid. 1997; 7:613–9.
Article8. NCSS. Chapter 210: Non-inferiority tests for the difference between two proportions [Internet]. Kaysville: NCSS;c2020. [cited 2020 Jul 13]. Available from: https://ncss-wpengine.netdna-ssl.com/wp-content/themes/ncss/pdf/Procedures/PASS/Non-Inferiority_Tests_for_the_Difference_Between_Two_Proportions.pdf.9. Jeon MJ, Yoon JH, Han JM, Yim JH, Hong SJ, Song DE, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol. 2013; 168:219–25.
Article10. Mallick U, Harmer C, Hackshaw A, Moss L; IoN Trial Management Group. Iodine or Not (IoN) for low-risk differentiated thyroid cancer: the next UK National Cancer Research Network randomised trial following HiLo. Clin Oncol (R Coll Radiol). 2012; 24:159–61.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postoperative Follow-Up of Differentiated Thyroid Cancer: Use of Thyroglobulin Assay
- Analysis of Urine Iodine Excretion Decrease by Two-Week Stringent Low Iodine Diet for Remnant Thyroid Ablation with Radioactive Iodine in Korean Patients with Thyroid Cancer; Prospective Study
- Clinical Outcome of Remnant Thyroid Ablation with Low Dose Radioiodine in Korean Patients with Low to Intermediate-risk Thyroid Cancer
- Diagnostic Whole-Body Scan May Not Be Necessary for Intermediate-Risk Patients with Differentiated Thyroid Cancer after Low-Dose (30 mCi) Radioactive Iodide Ablation
- Quality of Life in Patients with Papillary Thyroid Microcarcinoma According to Treatment: Total Thyroidectomy with or without Radioactive Iodine Ablation