Ann Lab Med.
2020 Nov;40(6):448-456. 10.3343/alm.2020.40.6.448.
Stability of pH, Blood Gas Partial Pressure, Hemoglobin Oxygen Saturation Fraction, and Lactate Concentration
- Affiliations
-
- 1Laboratori Clínic Territorial Metropolitana Sud–Hospital Universitari de Bellvitge. Hospitalet de Llobregat, Barcelona, Spain
- KMID: 2507790
- DOI: http://doi.org/10.3343/alm.2020.40.6.448
Abstract
- Background
The storage temperature and time of blood gas samples collected in syringes constitute preanalytical variables that could affect blood gas or lactate concentration measurement results. We analyzed the effect of storage temperature and time delay on arterial or venous blood gas stability related to pH, partial pressure of carbon dioxide (pCO2) and oxygen (pO2), hemoglobin oxygen saturation (sO2), and lactate concentration.
Methods
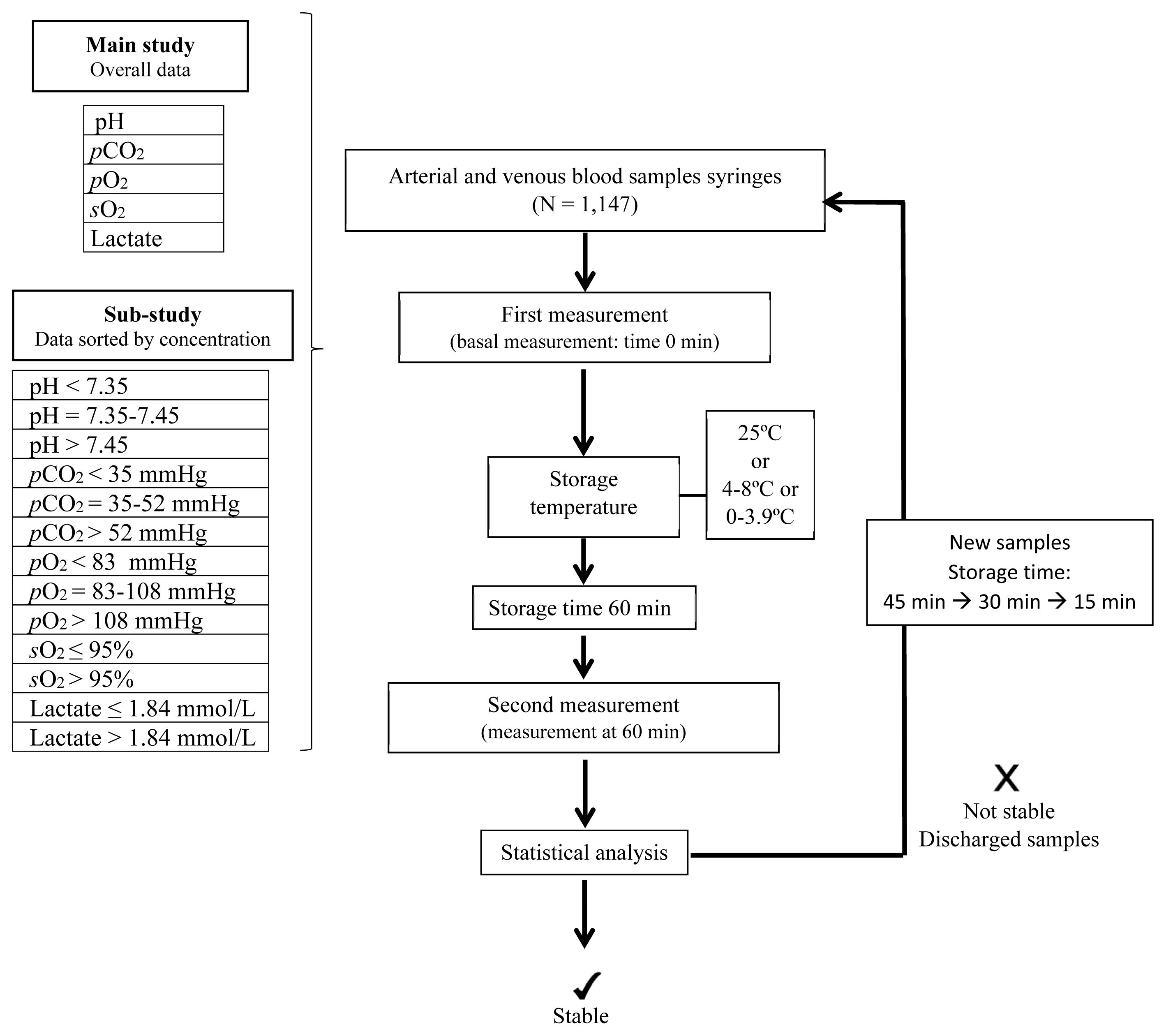
In total, 1,200 arterial and venous blood sample syringes were analyzed within 10 minutes of collection. The samples were divided into different groups to determine parameter stability at 25, 4–8, and 0–3.9°C and at different storage times, 60, 45, 30, and 15 minutes. Independent sample groups were used for each analysis. Percentage deviations were calculated and compared with acceptance stability limits (1.65 × coefficient of variation). Additionally, sample group sub analysis was performed to determine whether stability was concentration-dependent for each parameter.
Results
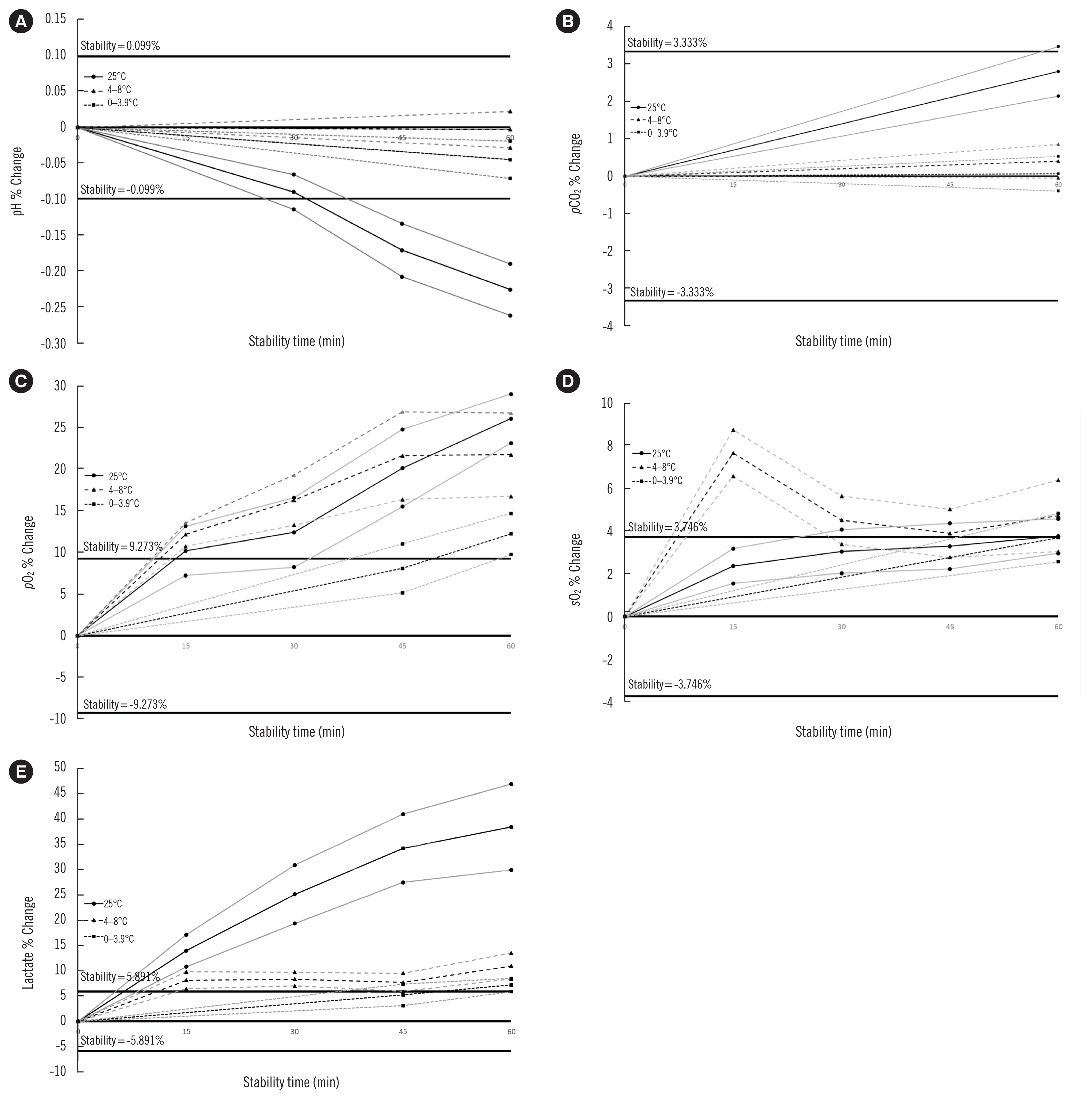
The pH was stable over all storage times at 4–8 and 0–3.9°C and up to 30 minutes at 25°C. pCO2 was stable at ≤ 60 minutes at all temperatures. pO2 was stable for 45 minutes at 0–3.9°C, and sO2 was stable for 15 minutes at 25°C and for ≤ 60 minutes at 0–3.9°C. Lactate concentration was stable for 45 minutes at 0–3.9°C. Subanalysis showed that stability was concentration-dependent.
Conclusions
The strictest storage temperature and time criteria (0–3.9°C, 45 minutes) should be adopted for measuring pH, pCO2, pO2, sO2, and lactate concentration in blood gas syringes.
Keyword
Figure
Cited by 3 articles
-
The Stability of Blood Gas Parameters Depends on Leukocyte Counts
Lucie Vaudran, Jean David Pekar, Guillaume Grzych, Patrice Maboudou
Ann Lab Med. 2021;41(5):502-503. doi: 10.3343/alm.2021.41.5.502.Reply to the Letter “The Stability of Blood Gas Parameters Depends on Leukocyte Count”
Ariadna Arbiol-Roca, Claudia Elizabeth Imperiali
Ann Lab Med. 2021;41(5):504-505. doi: 10.3343/alm.2021.41.5.504.Performance Evaluation of the i-SmartCare 10 Analyzer and Method Comparison of Six Point-of-Care Blood Gas Analyzers
Sang-Mi Kim, Hyung-Doo Park
Ann Lab Med. 2022;42(4):467-472. doi: 10.3343/alm.2022.42.4.467.
Reference
-
1. Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002; 48:691–8.
Article2. Thelen MHM, Huisman W. Harmonization of accreditation to ISO15189. Clin Chem Lab Med. 2018; 56:1637–43.
Article3. UNE-EN ISO 15189. 2012. Medical laboratories–particular requirements for quality and competence.4. AARC clinical practice guideline. Sampling for arterial blood gas analysis. American Association for Respiratory Care. Respir Care. 1992; 37:913–7.5. American Association for Respiratory Care. AARC Clinical Practice Guideline: blood gas analysis and hemoximetry: 2001 revision and update. Respir Care. 2001; 46:498–505.6. Burnett RW, Covington AK, Fogh-Andersen N, Külpmann WR, Maas AH, Müller-Plathe O, et al. International federation of clinical chemistry (IFCC). Scientific division. Committee on pH, blood gases and electrolytes. Approved IFCC recommendations on whole blood sampling, transport and storage for simultaneous determination of pH, blood gases and electrolytes. Eur J Clin Chem Clin Biochem. 1995; 33:247–53.7. Dukić L, Milevoj Kopčinović L, Dorotić A, Baršić I. Blood gas testing and related measurements: National recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med (Zagreb). 2016; 26:318–36.
Article8. Mahoney JJ, Harvey JA, Wong RJ, Van Kessel AL. Changes in oxygen measurements when whole blood is stored in iced plastic or glass syringes. Clin Chem. 1991; 37:1244–8.
Article9. Liss HP, Payne CP Jr. Stability of blood gases in ice and at room temperature. Chest. 1993; 103:1120–2.
Article10. Schmidt C, Müller-Plathe O. Stability of pO2, pCO2, and pH in heparinized whole blood samples: influence of storage temperature with regard to leukocyte count and syringe material. Eur J Clin Chem Clin Biochem. 1992; 30:767–73.
Article11. Beaulieu M, Lapointe Y, Vinet B. Stability of PO2, PCO2, and pH in fresh blood samples stored in a plastic syringe with low heparin in relation to various blood-gas and hematological parameters. Clin Biochem. 1999; 32:101–7.12. Smeenk FW, Janssen JD, Arends BJ, Harff GA, van den Bosch JA, Schönberger JP, et al. Effects of four different methods of sampling arterial blood and storage time on gas tensions and shunt calculation in the 100% oxygen test. Eur Respir J. 1997; 10:910–3.13. Pretto JJ, Rochford PD. Effects of sample storage time, temperature and syringe type on blood gas tensions in samples with high oxygen partial pressures. Thorax. 1994; 49:610–2.
Article14. Alsina MJ, González-Oller RK. SEQC Laboratory Quality Assurance and Accreditation Committee. Definition of the stability limit of the parameters in the biological samples. Química Clínica. 2006; 25:81–5. (article in Spanish).15. Ricós C, Cava F, García-Lario JV, Hernández A, Iglesias N, Jiménez CV, et al. The reference change value: A proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest. 2004; 64:175–84.
Article16. Smajić J, Kadić D, Hasić S, Serdarević N. Effects of post-sampling analysis time, type of blood samples and collection tubes on values of blood gas testing. Med Glas (Zenica). 2015; 12:108–12.17. Knowles TP, Mullin RA, Hunter JA, Douce FH. Effects of syringe material, sample storage time, and temperature on blood gases and oxygen saturation in arterialized human blood samples. Respir Care. 2006; 51:732–6.18. Srisan P, Udomsri T, Jetanachai P, Lochindarat S, Kanjanapattanakul W. Effects of temperature and time delay on arterial blood gas and electrolyte measurements. J Med Assoc Thai. 2011; 94(S3):S9–14.19. Williams AJ. ABC of oxygen: assessing and interpreting arterial blood gases and acid-base balance. BMJ. 1998; 317:1213–6.
Article20. Baird GB. Preanalytical considerations in blood gas analysis. Biochem Med (Zagreb). 2013; 23:19–27.
Article21. Arbiol-Roca A, Dot-Bach D. Critical issues and new trends on stat tests in clinical laboratory. EJIFCC. 2019; 30:59–66.22. Brazg J, Huang P, Weiner C, Singh G, Likourezos A, Salem L, et al. Relocation of blood gas laboratory to the emergency department helps decrease lactic acid values. Am J Emerg Med. 2018; 36:2035–7.
Article23. Gruber MA, Felbermeir S, Lindner R, Kieninger M. Preanalytics: the (in-)stability of volatile POCT parameters and the homogeneity of blood in syringes at the market. Clin Chim Acta. 2016; 457:18–23.
Article24. Mohammadhoseini E, Safavi E, Seifi S, Seifirad S, Firoozbakhsh S, Peiman S. Effect of sample storage temperature and time delay on blood gases, bicarbonate and pH in human arterial blood samples. Iran Red Crescent Med J. 2015; 17:e13577.
Article25. CLSI. Procedures for the collection of arterial blood specimens; approved standard. 4th ed. CLSI GP43-A4 (former H11-A4). Wayne, PA: Clinical and Laboratory Standards Institute;2004.26. Kieninger M, Zech N, Mulzer Y, Bele S, Seemann M, Künzig H, et al. Optimization of blood gas analysis in intensive care units: reduction of preanalytical errors and improvement of workflow. Anaesthesist. 2015; 64:365–72.27. Murphy R, Thethy S, Raby S, Beckley J, Terrace J, Fiddler C, et al. Capillary blood gases in acute exacerbations of COPD. Respir Med. 2006; 100:682–6.
Article28. Liu D, Li YX, Huang Y. Analysis of hemolysis, icterus and lipemia in arterial blood gas specimens. Clin Chem Lab Med. 2017; 55:e69–71.
Article29. Ruppel GL. Of time and temperature, plastic and glass: specimen handling in the blood-gas laboratory. Respir Care. 2006; 51:717–8.30. Davis MD, Walsh BK, Sittig SE, Restrepo RD. AARC clinical practice guideline: blood gas analysis and hemoximetry: 2013. Respir Care. 2013; 58:1694–703.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Gas Analysis in Arterial and Venous Blood during Inspiration of High Oxygen Concentration
- The Changes of Blood Lactate Concentrations during Open - heart Sugery
- Effects of Various F1O2 on Central and Mixed Venous Oxygen Saturation during Mechanical Ventilation
- The Adequate Timing of Arterial Blood Sampling during the Changes of Inspired Oxygen Fraction by Nitrous Oxide
- Effect of Time Interval on Arterial Blood Gas Findings