Anat Cell Biol.
2020 Sep;53(3):292-300. 10.5115/acb.19.241.
Role of cerebrospinal fluid in differentiation of human dental pulp stem cells into neuron-like cells
- Affiliations
-
- 1Department of Brain and Cognitive Sciences, Cell Science Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran
- 2Department of Anatomy & Cell Biology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 3Immunogenetic Research Center, Department of Anatomy & Cell Biology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 4Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 5Research Laboratory for Embryology and Stem Cells, Department of Anatomical Sciences, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran
- 6Department of Anatomical Sciences, School of Medical Sciences, Bushehr University of Medical Sciences, Bushehr, Iran
- 7Abadan School of Medical Sciences, Abadan, Iran
- 8Cellular and Molecular Research Center, Qazvin University of Medical Sciences, Qazvin, Iran
- KMID: 2507642
- DOI: http://doi.org/10.5115/acb.19.241
Abstract
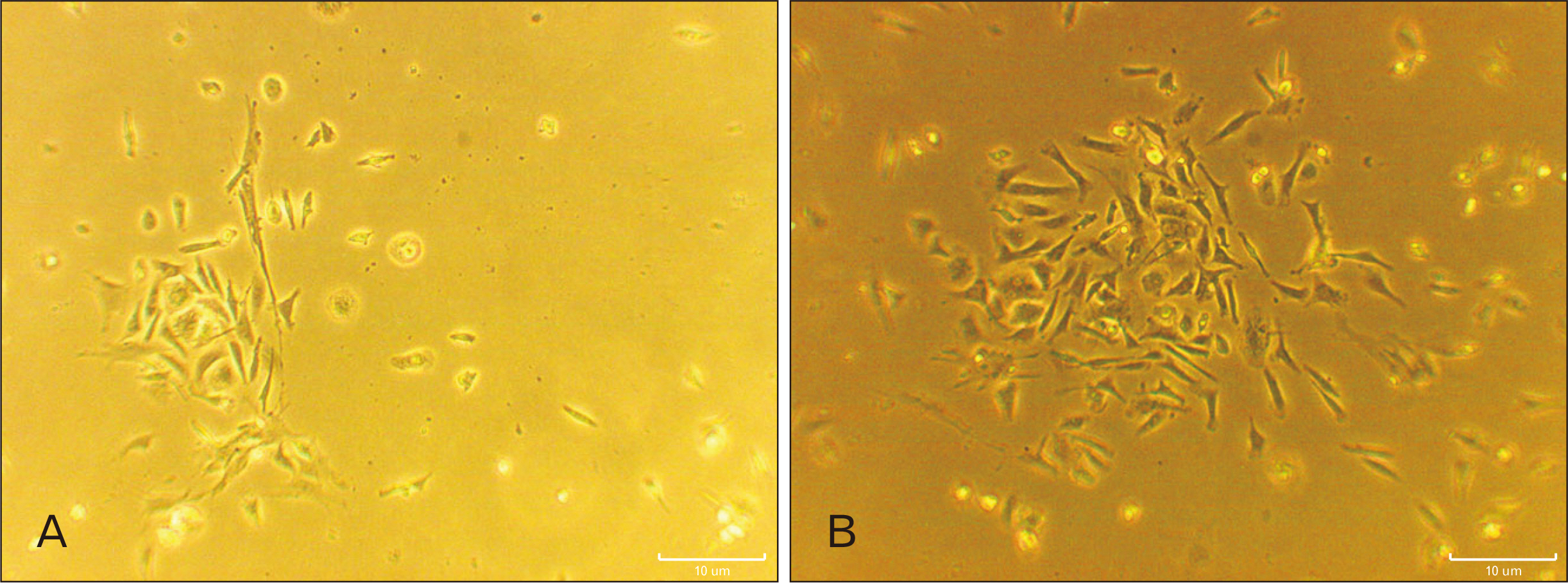
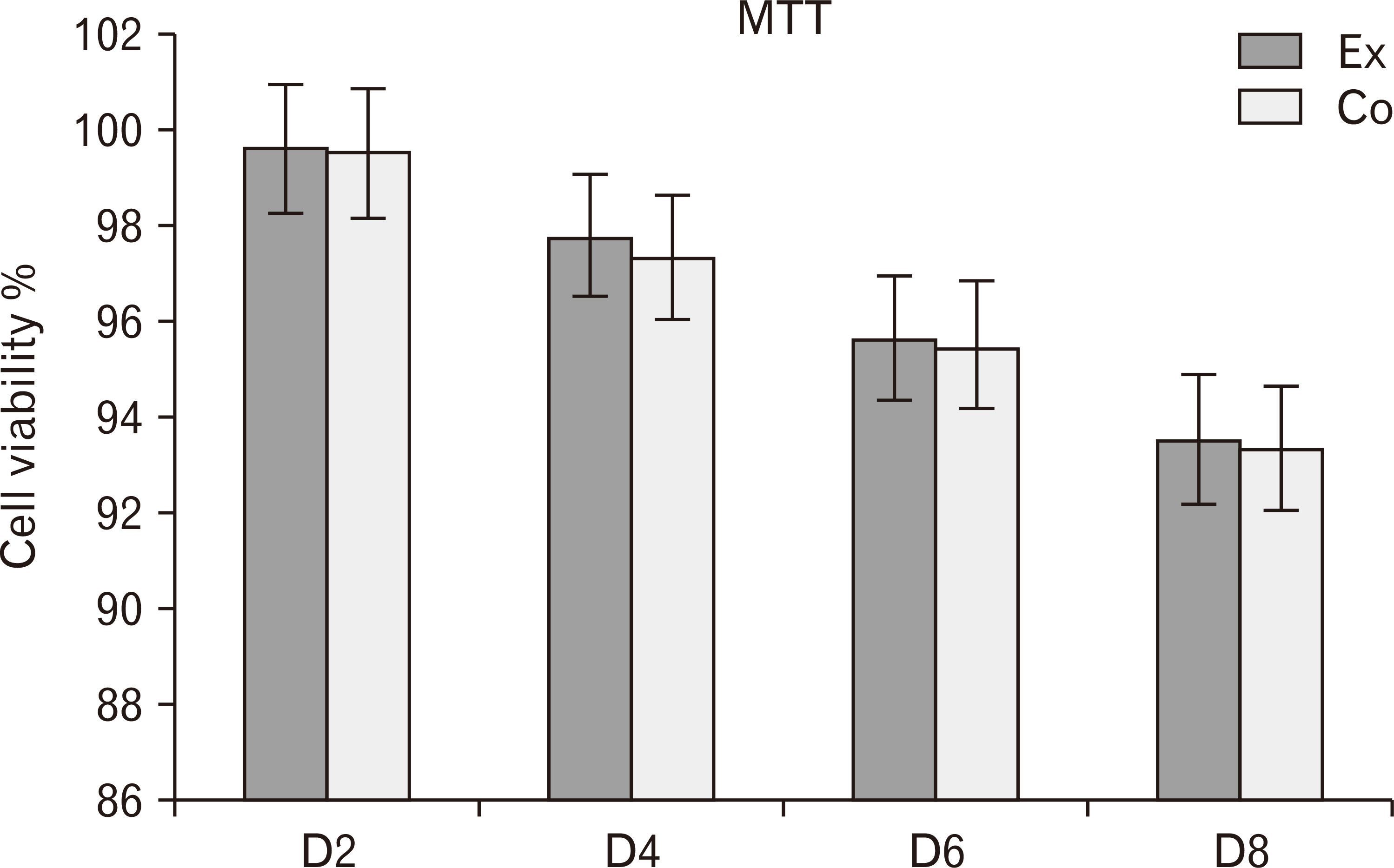
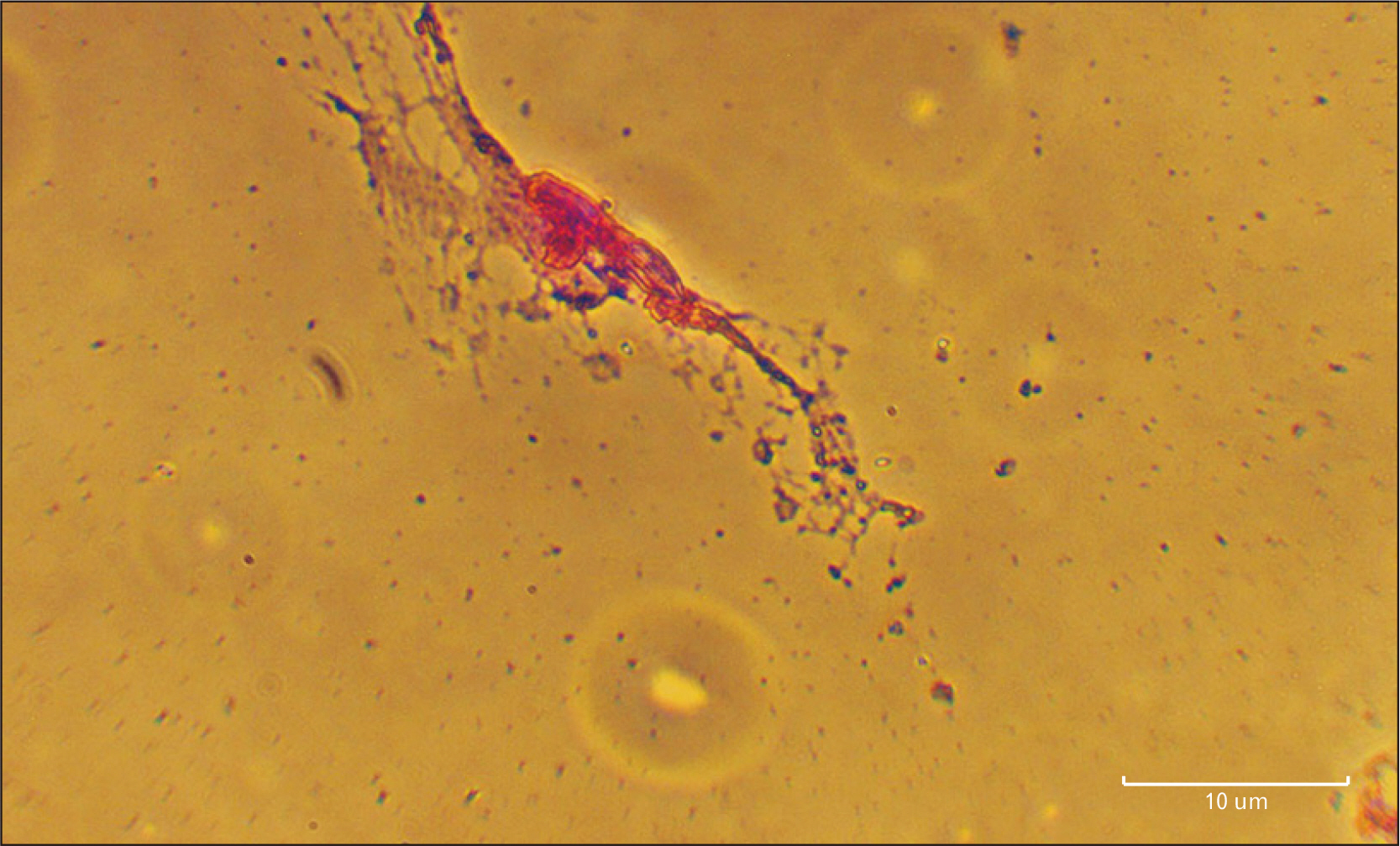
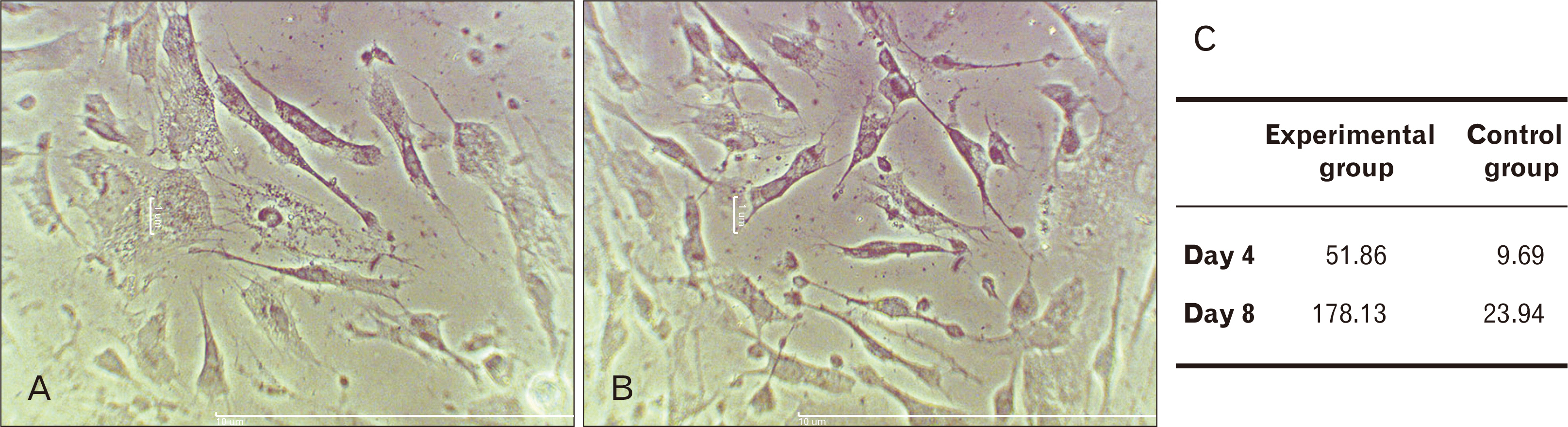
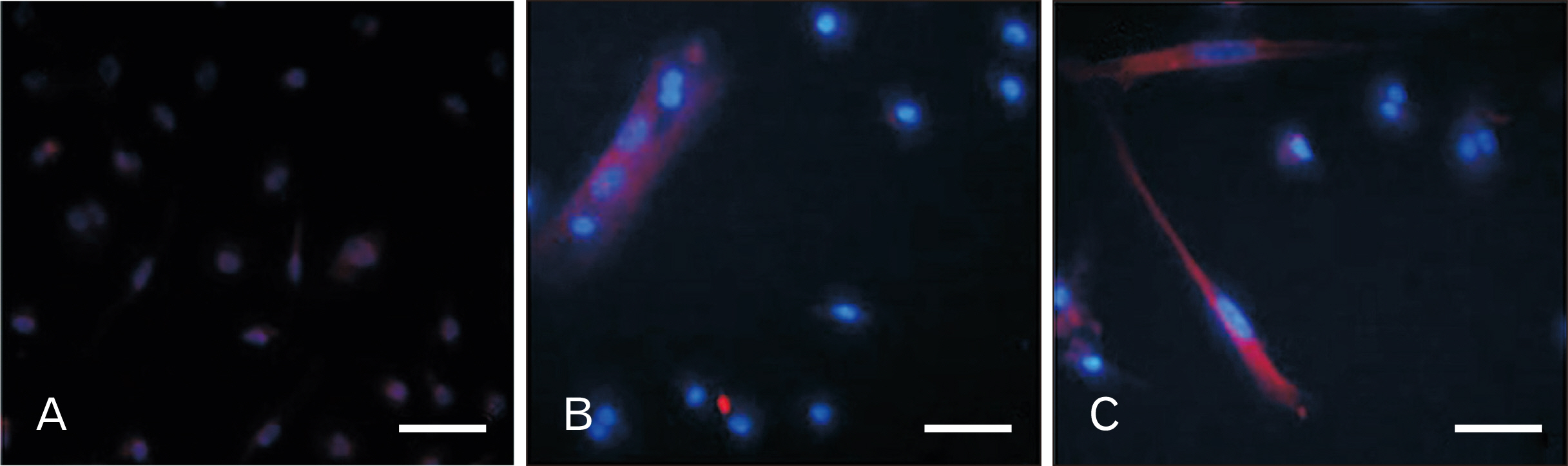
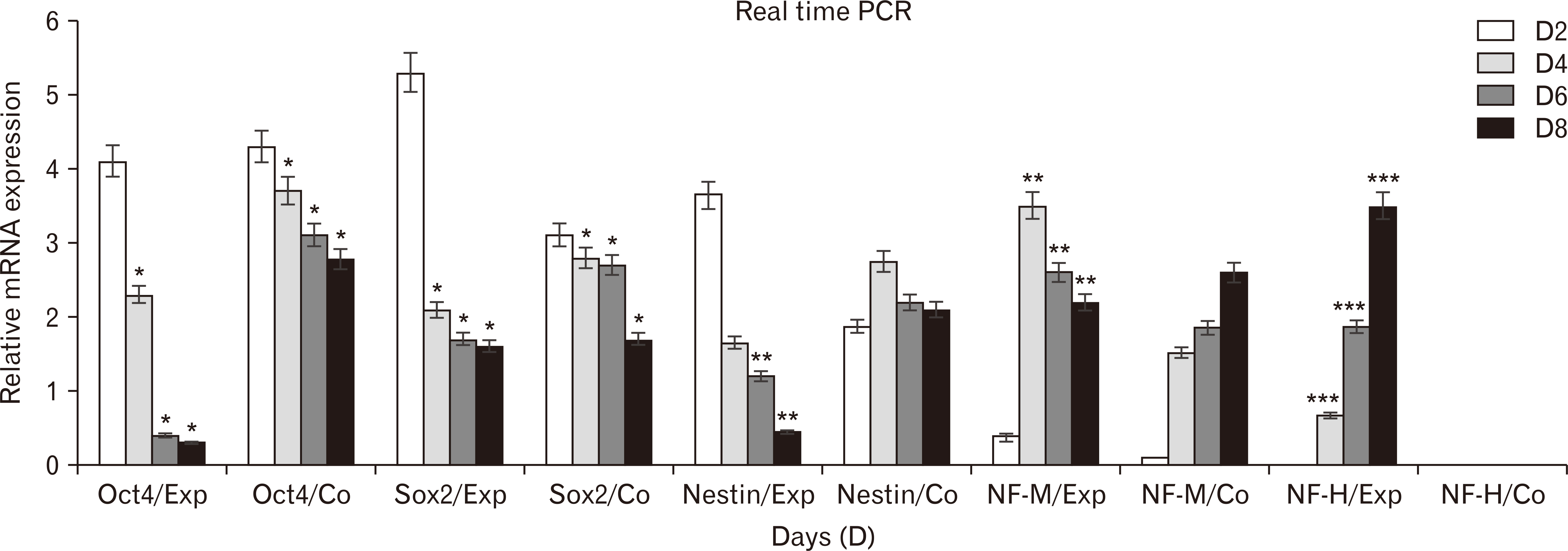
- Human dental pulp stem cells (hDPSCs) could be differentiated into neuron like-cells under particular microenvironments. It has been reported that a wide range of factors, presented in cerebrospinal fluid (CSF), playing part in neuronal differentiation during embryonic stages, we herein introduce a novel culture media complex to differentiate hDPSCs into neuron-like cells. The hDPSCs were initially isolated and characterized. The CSF was prepared from the Cisterna magna of 19-day-old Wistar rat embryos, embryonic cerebrospinal fluid (E-CSF). The hDPSCs were treated by 5% E-CSF for 2 days, then neurospheres were cultured in DMEM/F12 supplemented with 10-6 μm retinoic acid (RA), glialderived neurotrophic factor and brain-derived neurotrophic factor for 6 days. The cells which were cultured in basic culture medium were considered as control group. Morphology of differentiated cells as well as process elongation were examined by an inverted microscope. In addition, the neural differentiation markers (Nestin and MAP2) were studied employing immunocytochemistry. Neuronal-like processes appeared 8 days after treatment. Neural progenitor marker (Nestin) and a mature neural marker (MAP2) were expressed in treated group. Moreover Nissl bodies were found in the cytoplasm of treated group. Taking these together, we have designed a simple protocol for generating neuron-like cells using CSF from the hDPSCs, applicable for cell therapy in several neurodegenerative disorders including Alzheimer’s disease.
Figure
Cited by 1 articles
-
Role of agmatine in the application of neural progenitor cell in central nervous system diseases: therapeutic potentials and effects
Renée Kosonen, Sumit Barua, Jong Youl Kim, Jong Eun Lee
Anat Cell Biol. 2021;54(2):143-151. doi: 10.5115/acb.21.089.
Reference
-
References
1. Shokohi R, Nabiuni M, Irian S, Miyan JA. 2018; In vitro effects of wistar rat prenatal and postnatal cerebrospinal fluid on neural differentiation and proliferation of mesenchymal stromal cells derived from bone marrow. Cell J. 19:537–44. DOI: 10.1590/1678-4324-2017160221. PMID: 29105387. PMCID: PMC5672091.2. Pandamooz S, Naji M, Alinezhad F, Zarghami A, Pourghasem M. 2013; The influence of cerebrospinal fluid on epidermal neural crest stem cells may pave the path for cell-based therapy. Stem Cell Res Ther. 4:84. DOI: 10.1186/scrt235. PMID: 23867009. PMCID: PMC3854676.
Article3. Ren C, Yin P, Ren N, Wang Z, Wang J, Zhang C, Ge W, Geng D, Wang X. 2018; Cerebrospinal fluid-stem cell interactions may pave the path for cell-based therapy in neurological diseases. Stem Cell Res Ther. 9:66. DOI: 10.1186/s13287-018-0807-3. PMID: 29523182. PMCID: PMC5845187.
Article4. Goudarzi G, Hamidabadi HG, Abdanipour A, Kouchesfehani HM, Moghaddam AE. 2016; The influence of cerebrospinal fluid accompanied by retinoic acid on differentiation of bone marrow mesenchymal stem cells into neuron-like cells in vitro. Pathobiol Res. 18:79–91.5. Ziegler AN, Levison SW, Wood TL. 2015; Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol. 11:161–70. DOI: 10.1038/nrendo.2014.208. PMID: 25445849. PMCID: PMC5513669.
Article6. Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D'Ercole AJ, Wong ET, LaMantia AS, Walsh CA. 2011; The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 69:893–905. DOI: 10.1016/j.neuron.2011.01.023. PMID: 21382550. PMCID: PMC3085909.
Article7. Polanco JC, Li C, Bodea LG, Martinez-Marmol R, Meunier FA, Götz J. 2018; Amyloid-β and tau complexity- towards improved biomarkers and targeted therapies. Nat Rev Neurol. 14:22–39. DOI: 10.1038/nrneurol.2017.162. PMID: 29242522.8. Kondo A, Shahpasand K, Mannix R, Qiu J, Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, Wei S, Luo ML, Albayram O, Huang P, Rotenberg A, Ryo A, Goldstein LE, Pascual-Leone A, McKee AC, Meehan W, Zhou XZ, Lu KP. 2015; Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 523:431–6. DOI: 10.1038/nature14658. PMID: 26176913. PMCID: PMC4718588.
Article9. Chang CC, Chang KC, Tsai SJ, Chang HH, Lin CP. 2014; Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J Formos Med Assoc. 113:956–65. DOI: 10.1016/j.jfma.2014.09.003. PMID: 25438878.
Article10. Haratizadeh S, Nazm Bojnordi M, Darabi S, Karimi N, Naghikhani M, Ghasemi Hamidabadi H, Seifi M. 2017; Condition medium of cerebrospinal fluid and retinoic acid induces the transdifferentiation of human dental pulp stem cells into neuroglia and neural like cells. Anat Cell Biol. 50:107–14. DOI: 10.5115/acb.2017.50.2.107. PMID: 28713614. PMCID: PMC5509894.
Article11. Bojnordi MN, Haratizadeh S, Darabi S, Hamidabadi HG. 2018; Neural derivation of human dental pulp stem cells via neurosphere technique. Bratisl Lek Listy. 119:550–3. DOI: 10.4149/BLL_2018_099. PMID: 30226064.
Article12. Bain G, Ray WJ, Yao M, Gottlieb DI. 1996; Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem Biophys Res Commun. 223:691–4. DOI: 10.1006/bbrc.1996.0957. PMID: 8687458.
Article13. Nazm Bojnordi M, Movahedin M, Tiraihi T, Javan M, Ghasemi Hamidabadi H. 2014; Oligoprogenitor cells derived from spermatogonia stem cells improve remyelination in demyelination model. Mol Biotechnol. 56:387–93. DOI: 10.1007/s12033-013-9722-0. PMID: 24282061.
Article14. Sagha M, Esfandiari E, Razavi S, Tanhaee S, Nasr-Esfahani MH, Baharvand H. 2013; Role of retinoic acid in neural patterning of mouse embryonic stem cells. Arak Med Univ J. 16:16–26.15. Mahmoudinia S, Niapour A, Ghasemi Hamidabadi H, Mazani M. 2019; 2,4-D causes oxidative stress induction and apoptosis in human dental pulp stem cells (hDPSCs). Environ Sci Pollut Res Int. 26:26170–83. DOI: 10.1007/s11356-019-05837-0. PMID: 31280441.
Article16. Gato A, Moro JA, Alonso MI, Bueno D, De La Mano A, Martín C. 2005; Embryonic cerebrospinal fluid regulates neuroepithelial survival, proliferation, and neurogenesis in chick embryos. Anat Rec A Discov Mol Cell Evol Biol. 284:475–84. DOI: 10.1002/ar.a.20185. PMID: 15803475.
Article17. Nabiuni M, Shokohi R, Moghaddam P. 2015; CSF protein contents and their roles in brain development. Zahedan J Res Med Sci. 17:e1042. DOI: 10.17795/zjrms-1042.
Article18. Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M. 2013; A new multistep induction protocol for the transdifferentiation of bone marrow stromal stem cells into GABAergic neuron-like cells. Iran Biomed J. 17:8–14. DOI: 10.6091/ibj.1112.2012. PMID: 23279829. PMCID: PMC3600975.19. Youssef AR, Emara R, Taher MM, Al-Allaf FA, Almalki M, Almasri MA, Siddiqui SS. 2019; Effects of mineral trioxide aggregate, calcium hydroxide, biodentine and Emdogain on osteogenesis, Odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral Health. 19:133. DOI: 10.1186/s12903-019-0827-0. PMID: 31266498. PMCID: PMC6604301.
Article20. Cao Q, Benton RL, Whittemore SR. 2002; Stem cell repair of central nervous system injury. J Neurosci Res. 68:501–10. DOI: 10.1002/jnr.10240. PMID: 12111840.
Article21. Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, Ghetti B, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Salloway S, Schofield PR, Sperling RA, Marcus D, Cairns NJ, Buckles VD, Ladenson JH, Morris JC, Holtzman DM. 2014; Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease. Sci Transl Med. 6:226ra30. DOI: 10.1126/scitranslmed.3007901. PMID: 24598588.
Article22. Alipour M, Nabavi SM, Arab L, Vosough M, Pakdaman H, Ehsani E, Shahpasand K. 2019; Stem cell therapy in Alzheimer's disease: possible benefits and limiting drawbacks. Mol Biol Rep. 46:1425–46. DOI: 10.1007/s11033-018-4499-7. PMID: 30565076.
Article23. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000; Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 97:13625–30. DOI: 10.1073/pnas.240309797. PMID: 11087820. PMCID: PMC17626.24. Parr AM, Tator CH, Keating A. 2007; Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 40:609–19. DOI: 10.1038/sj.bmt.1705757. PMID: 17603514. PMCID: PMC5716453.
Article25. Kim SH, Oh KW, Jin HK, Bae JS. 2018; Immune inflammatory modulation as a potential therapeutic strategy of stem cell therapy for ALS and neurodegenerative diseases. BMB Rep. 51:545–6. DOI: 10.5483/BMBRep.2018.51.11.255. PMID: 30463642. PMCID: PMC6283021.
Article26. Sanchez-Ramos JR. 2002; Neural cells derived from adult bone marrow and umbilical cord blood. J Neurosci Res. 69:880–93. DOI: 10.1002/jnr.10337. PMID: 12205681. PMCID: PMC7036219.
Article27. Wang F, Jia Y, Liu J, Zhai J, Cao N, Yue W, He H, Pei X. 2017; Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer's disease. Cell Biol Int. 41:639–50. DOI: 10.1002/cbin.10767. PMID: 28328017.
Article28. Zhang W, Wang PJ, Sha HY, Ni J, Li MH, Gu GJ. 2014; Neural stem cell transplants improve cognitive function without altering amyloid pathology in an APP/PS1 double transgenic model of Alzheimer's disease. Mol Neurobiol. 50:423–37. DOI: 10.1007/s12035-014-8640-x. PMID: 24481678.
Article29. Hens J, Nuydens R, Geerts H, Senden NH, Van de Ven WJ, Roebroek AJ, van de Velde HJ, Ramaekers FC, Broers JL. 1998; Neuronal differentiation is accompanied by NSP-C expression. Cell Tissue Res. 292:229–37. DOI: 10.1007/s004410051054. PMID: 9560466. PMCID: PMC6824316.
Article30. Chen Y, Teng FY, Tang BL. 2006; Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation: progress and uncertainties. Cell Mol Life Sci. 63:1649–57. DOI: 10.1007/s00018-006-6019-5. PMID: 16786223.
Article31. Reitz C, Brayne C, Mayeux R. 2011; Epidemiology of Alzheimer disease. Nat Rev Neurol. 7:137–52. DOI: 10.1038/nrneurol.2011.2. PMID: 21304480.
Article32. Haratizadeh S, Bojnordi MN, Niapour A, Bakhtiari M, Hamidabadi HG. 2016; Improvement of neuroglial differentiation from human dental pulp stem cells using CSF. Majallahi Danishgahi Ulumi Pizishkii Mazandaran. 26:1–14.33. Lehtinen MK, Bjornsson CS, Dymecki SM, Gilbertson RJ, Holtzman DM, Monuki ES. 2013; The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J Neurosci. 33:17553–9. DOI: 10.1523/JNEUROSCI.3258-13.2013. PMID: 24198345. PMCID: PMC3818536.
Article34. Silvagno F, Guarnieri V, Capizzi A, Pescarmona GP. 2002; Synergistic effect of retinoic acid and dehydroepiandrosterone on differentiation of human neuroblastoma cells. FEBS Lett. 532:153–8. DOI: 10.1016/S0014-5793(02)03667-0. PMID: 12459481.
Article35. Yung SY, Gokhan S, Jurcsak J, Molero AE, Abrajano JJ, Mehler MF. 2002; Differential modulation of BMP signaling promotes the elaboration of cerebral cortical GABAergic neurons or oligodendrocytes from a common sonic hedgehog-responsive ventral forebrain progenitor species. Proc Natl Acad Sci U S A. 99:16273–8. DOI: 10.1073/pnas.232586699. PMID: 12461181. PMCID: PMC138601.
Article36. Liu JP, Laufer E, Jessell TM. 2001; Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 32:997–1012. DOI: 10.1016/S0896-6273(01)00544-X. PMID: 11754833.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Characterization of Human Dental Pulp Cells from Supernumerary Teeth by Using Flow Cytometry Analysis
- Dental Pulp Stem Cells and Current in vivo Approaches to Study Dental Pulp Stem Cells in Pulp Injury and Regeneration
- Neurogenic differentiation of human dental stem cells in vitro
- Effects of nanoscale ridge/groovepattern arrayed surface on in vitro differentiation of multi-potent pulp cells derived from human supernumerary teeth