Infect Chemother.
2020 Mar;52(1):48-58. 10.3947/ic.2020.52.1.48.
Differences in Vancomycin Clearance between Trauma and Medical Intensive Care Unit Patients
- Affiliations
-
- 1Department of Infectious Diseases, Ajou University School of Medicine, Suwon, Korea
- 2Department of Pharmacy, Ajou University Medical Center, Suwon, Korea
- 3Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, Korea
- KMID: 2507323
- DOI: http://doi.org/10.3947/ic.2020.52.1.48
Abstract
- Background
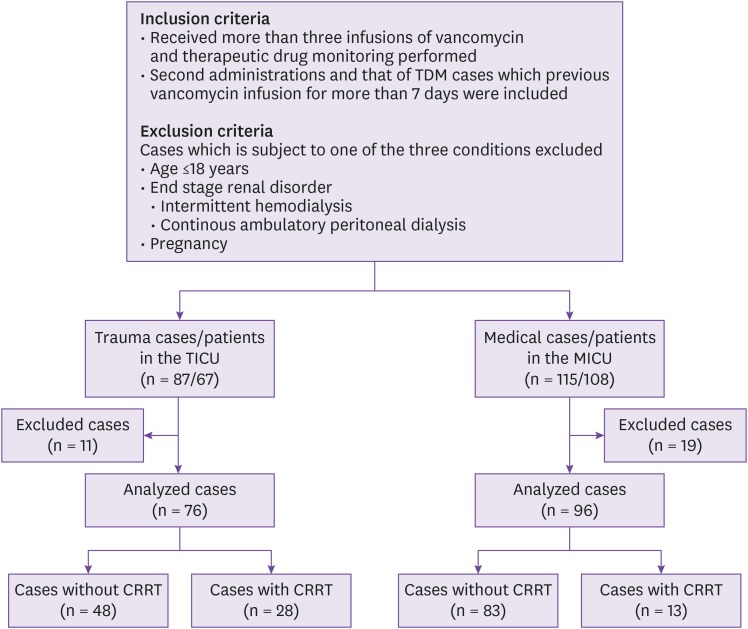
To identify the differences in the vancomycin pharmacokinetics between multiple trauma patients and medically ill patients in the intensive care unit (ICU) stratified by the use of continuous renal replacement therapy (CRRT), and the factors affecting vancomycin clearance (CLvan ).
Materials and Methods
All the included patients received at least three consecutive doses of vancomycin, then, therapeutic drug monitoring was conducted. Patients' serum vancomycin trough levels and other clinical variables were identified retrospectively. The vancomycin pharmacokinetics and associated factors were compared and analyzed between trauma ICU (TICU) and medical ICU (MICU) patients.
Results
In the non-dialyzed group, the CLvan was higher among the TICU patients than the MICU patients. However, in the continuous renal replacement therapy group, there was no significant difference in the CLvan between the multiple trauma and medically ill patients. The only factor associated with CLvan in the non-dialyzed group was creatinine clearance; none of the factors was associated with CLvan in the CRRT group.
Conclusion
In the case of non-dialyzed patients in the TICU, vancomycin dosages must be adjusted, depending on the patient's actual body weight changes. In the case of patients undergoing CRRT in both ICUs, vancomycin can be infused with fixed doses regardless of the patients' characteristics.
Keyword
Figure
Reference
-
1. Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. 2006; 12:325–332. PMID: 16810043.
Article2. Sedlář M, Kvasnička J, Krška Z, Tománková T, Linhart A. Early and subacute inflammatory response and long-term survival after hip trauma and surgery. Arch Gerontol Geriatr. 2015; 60:431–436. PMID: 25704919.
Article3. Bunnell KL, Zullo AR, Collins C, Adams CA Jr. Methicillin-resistant Staphylococcus aureus pneumonia in critically ill trauma and burn patients: a retrospective cohort study. Surg Infect (Larchmt). 2017; 18:196–201. PMID: 28004983.4. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009; 49:325–327. PMID: 19569969.
Article5. McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother. 2011; 66(Suppl 2):ii25–ii31. PMID: 21398304.
Article6. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006; 42(Suppl 1):S35–S39. PMID: 16323118.
Article7. Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr., Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009; 66:82–98. PMID: 19106348.
Article8. Scaglione F, Paraboni L. Pharmacokinetics/pharmacodynamics of antibacterials in the intensive care unit: setting appropriate dosing regimens. Int J Antimicrob Agents. 2008; 32:294–301. PMID: 18621508.
Article9. Pea F, Porreca L, Baraldo M, Furlanut M. High vancomycin dosage regimens required by intensive care unit patients cotreated with drugs to improve haemodynamics following cardiac surgical procedures. J Antimicrob Chemother. 2000; 45:329–335. PMID: 10702552.
Article10. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16:31–41. PMID: 1244564.
Article11. Mahmoud SH, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics. 2017; 9:E36. PMID: 28926966.
Article12. RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009; 361:1627–1638. PMID: 19846848.13. Baker SP, O'Neill B, Haddon W Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974; 14:187–196. PMID: 4814394.14. Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR Jr.SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med. 2005; 31:1336–1344. PMID: 16132893.
Article15. Ghosh SK. Basics of Bayesian methods. Methods Mol Biol. 2010; 620:155–178. PMID: 20652503.
Article16. Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010; 49:1–16. PMID: 20000886.17. Lin Wu FL, Liu SS, Yang TY, Win MF, Lin SW, Huang CF, Wang KC, Shen LJ. A larger dose of vancomycin is required in adult neurosurgical intensive care unit patients due to augmented clearance. Ther Drug Monit. 2015; 37:609–618. PMID: 25627406.
Article18. Medellín-Garibay SE, Ortiz-Martín B, Rueda-Naharro A, García B, Romano-Moreno S, Barcia E. Pharmacokinetics of vancomycin and dosing recommendations for trauma patients. J Antimicrob Chemother. 2016; 71:471–479. PMID: 26568565.
Article19. Minville V, Asehnoune K, Ruiz S, Breden A, Georges B, Seguin T, Tack I, Jaafar A, Saivin S, Fourcade O, Samii K, Conil JM. Increased creatinine clearance in polytrauma patients with normal serum creatinine: a retrospective observational study. Crit Care. 2011; 15:R49. PMID: 21291554.
Article20. Campassi ML, Gonzalez MC, Masevicius FD, Vazquez AR, Moseinco M, Navarro NC, Previgliano L, Rubatto NP, Benites MH, Estenssoro E, Dubin A. [Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment]. Rev Bras Ter Intensiva. 2014; 26:13–20. PMID: 24770684.
Article21. De Waele JJ, Dumoulin A, Janssen A, Hoste EA. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol. 2015; 81:1079–1085. PMID: 25697881.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Influencing Level of Awareness and Compliance with Vancomycin-Resistant Enterococcus Infection Control among Nurses in Intensive Care Units
- Development of a model to predict vancomycin serum concentration during continuous infusion of vancomycin in critically ill pediatric patients
- A Meta-Analysis on the Performance of Cystatin C- versus Creatinine-based eGFR Equations in Predicting Vancomycin Clearance
- Contributing Factors on Pharmacokinetic Variability in Critically Ill Neonates
- Usefulness of serum cystatin C to determine the dose of vancomycin in neonate