J Cerebrovasc Endovasc Neurosurg.
2020 Sep;22(3):165-175. 10.7461/jcen.2020.22.3.165.
Microsurgical treatment for the recurrent cerebral aneurysm initially treated using coil embolization
- Affiliations
-
- 1Department of Neurosurgery, Busan Paik Hospital, Inje University School of Medicine, Busan, Korea
- 2Department of Radiology, Busan Paik Hospital, Inje University School of Medicine, Busan, Korea
- 3Department of Endocrinology, Haeundae Paik Hospital, Inje University School of Medicine, Busan, Korea
- KMID: 2506763
- DOI: http://doi.org/10.7461/jcen.2020.22.3.165
Abstract
Objective
Microsurgical treatment could be a good alternative for the treatment of recurrent cerebral aneurysm after coil embolization. The purpose of this study was to present our experience of microsurgical treatment for recurrent cerebral aneurysm previously treated using coil embolization.
Methods
From June 2012 to May 2019, 34 patients consecutively received microsurgical treatment for a recurrent cerebral aneurysm previously treated using coil embolization after it ruptured.
Results
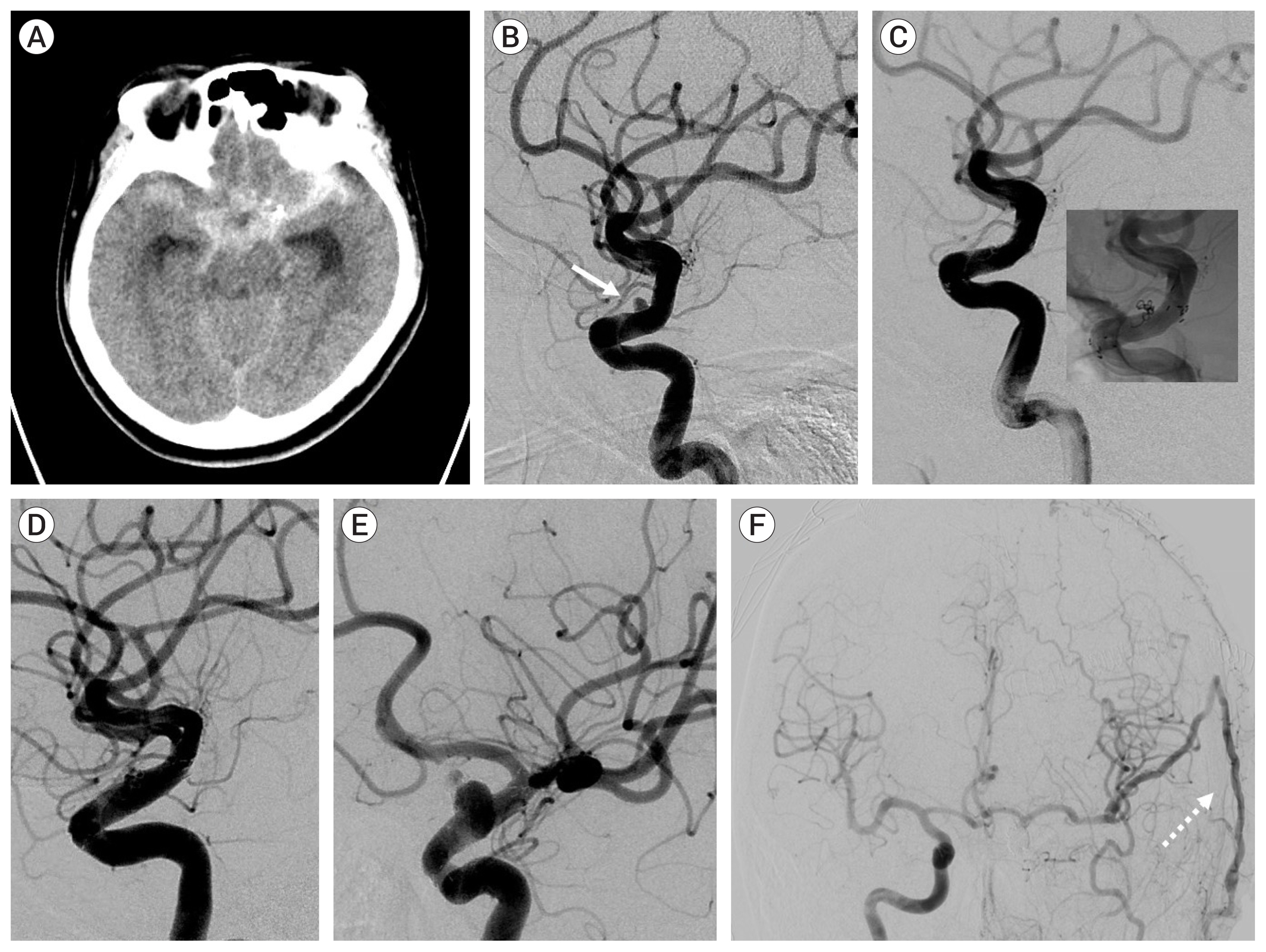
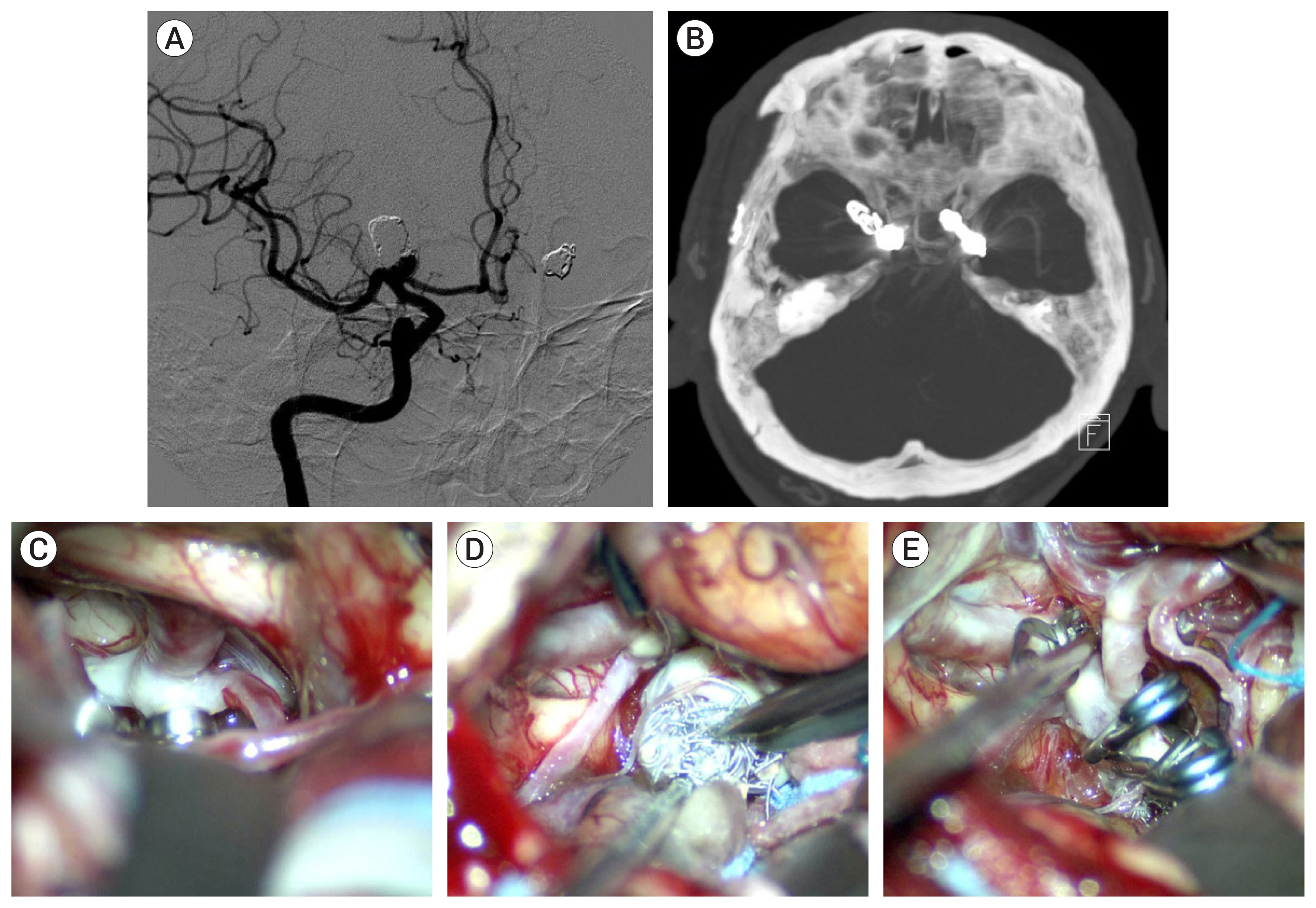
Of the 34 patients with aneurysm, 33 had the aneurysm located in the anterior circulation. The most common location was the anterior communicating artery (13 cases). Immediate radiologic outcome at coil embolization was completed (n=6), residual neck (n=26), and residual sac (n=2). The reason for microsurgical treatment included rebleeding (n=12), persistent residual sac (n=1), and recurrence on follow-up study (n=21). Rebleeding occurred within 10 days after coil embolization in 10 cases, and the other 2 were due to regrowth. In the 20 recurred and saccular aneurysms, coil compaction was present in 11 aneurysms and regrowth in 9 aneurysms. Simple neck clipping (n=29) and clipping with coil mass extraction (n=3) was possible in the saccular aneurysms. The blood blister like aneurysm (n=2) were treated using bypass and endovascular internal carotid artery trapping. In the follow-up study group after microsurgical treatment there were no severe complications due to the treatment. Age, cause of retreatment, and modified Rankin Scale before microsurgery were associated with good outcome (p<0.001).
Conclusions
Microsurgical treatment may be a viable and effective option for treating recurrent aneurysms previously treated by endovascular techniques.
Keyword
Figure
Cited by 1 articles
-
Microsurgical management of previously embolized intracranial aneurysms: A single center experience and literature review
Vasileios Panagiotopoulos, Ioannis Panagiotis Athinodorou, Kyprianos Kolios, Constantinos Kattou, Andreas Grzeczinski, Andreas Theofanopoulos, Lambros Messinis, Constantine Constantoyannis, Petros Zampakis
J Cerebrovasc Endovasc Neurosurg. 2025;27(1):1-18. doi: 10.7461/jcen.2024.E2024.05.004.
Reference
-
1. Ahn JY, Kim ST, Yi KC, Lee WH, Paeng SH, Jeong YG. Superficial temporal artery-sparing mini-pterional approach for cerebral aneurysm surgery. J Korean Neurosurg Soc. 2017; Jan. 60(1):8–14.
Article2. Arnaout OM, El Ahmadieh TY, Zammar SG, El Tecle NE, Hamade YJ, Aoun RJN, et al. Microsurgical treatment of previously coiled intracranial aneurysms: Systematic review of the literature. World Neurosurg. 2015; Aug. 84(2):246–53.
Article3. Bijlenga P, Morita A, Ko NU, Mocco J, Morel S, Murayama Y, et al. Common data elements for subarachnoid hemorrhage and unruptured intracranial aneurysms: Recommendations from the working group on subject characteristics. Neurocrit Care. 2019; Jun. 30(Suppl 1):20–27.
Article4. CARAT Investigators. Rates of delayed rebleeding from intracranial aneurysms are low after surgical and endovascular treatment. Stroke. 2006; Jun. 37(6):1437–42.5. Cho YD, Lee JY, Seo JH, Kang HS, Kim JE, Kwon OK, et al. Early recurrent hemorrhage after coil embolization in ruptured intracranial aneurysms. Neuroradiology. 2012; Jul. 54(7):719–26.
Article6. Dorfer C, Gruber A, Standhardt H, Bavinzski G, Knosp E. Management of residual and recurrent aneurysms after initial endovascular treatment. Neurosurgery. 2012; Mar. 70(3):537–53. discussion 553–4.
Article7. Izumo T, Matsuo T, Morofuji Y, Hiu T, Horie N, Hayashi K, et al. Microsurgical clipping for recurrent aneurysms after initial endovascular coil embolization. World Neurosurg. 2015; Feb. 83(2):211–8.
Article8. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR, et al. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: The cerebral aneurysm rerupture after treatment (CARAT) study. Stroke. 2008; Jan. 39(1):120–5.9. Kim ST, Baek JW, Jin SC, Park JH, Kim JS, Kim HY, et al. Coil embolization in patients with recurrent cerebral aneurysms who previously underwent surgical clipping. AJNR Am J Neuroradiol. 2019; Jan. 40(1):116–21.
Article10. Kim ST, Baek JW, Lee WH, Lee KS, Kwon WH, Pyo S, et al. Causes of early rebleeding after coil embolization of ruptured cerebral aneurysms. Clin Neurol Neurosurg. 2018; Nov. 174:108–16.
Article11. Lawton MT, Lang MJ. The future of open vascular neurosurgery: Perspectives on cavernous malformations, AVMs, and bypasses for complex aneurysms. J Neurosurg. 2019; May. 130(5):1409–25.
Article12. Meling TR. What are the treatment options for blister-like aneurysms? Neurosurg Rev. 2017; Oct. 40(4):587–93.
Article13. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the international subarachnoid aneurysm trial (ISAT). Lancet. 2015; Feb. 385(9969):691–7.
Article14. Murayama Y, Arakawa H, Ishibashi T, Kawamura D, Ebara M, Irie K, et al. Combined surgical and endovascular treatment of complex cerebrovascular diseases in the hybrid operating room. J Neurointerv Surg. 2013; Sep. 5(5):489–93.
Article15. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; Jun. 34(6):1398–403.
Article16. Romani R, Lehto H, Laakso A, Horcajadas A, Kivisaari R, von und zu Fraunberg M, et al. Microsurgery for previously coiled aneurysms: Experience with 81 patients. Neurosurgery. 2011; Jan. 68(1):140–53. discussion 153–4.
Article17. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001; Sep. 32(9):1998–2004.
Article18. Shtaya A, Dasgupta D, Millar J, Sparrow O, Bulters D, Duffill J. Outcomes of microsurgical clipping of recurrent aneurysms after endovascular coiling. World Neurosurg. 2018; Apr. 112:e540–7.
Article19. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P, et al. Ten-year analysis of saccular aneurysms in the barrow ruptured aneurysm trial. J Neurosurg. 2020; Mar. 132(3):771–6.
Article20. Toyota S, Kumagai T, Goto T, Mori K, Taki T. Clipping of recurrent cerebral aneurysms after coil embolization. Acta Neurochir Suppl. 2018; 129:53–9.
Article21. Waldron JS, Halbach VV, Lawton MT. Microsurgical management of incompletely coiled and recurrent aneurysms: Trends, techniques, and observations on coil extrusion. Neurosurgery. 2009; May. 64(5 Suppl 2):301–15. discussion 315–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Endovascular and Microsurgical Procedures as Complementary Approaches in the Treatment of a Single Intracranial Aneurysm
- Endovascular Coil Embolization After Clipping: Endovascular Treatment of Incompletely Clipped or Recurred Cerebral Aneurysms
- Recent Trends in the Treatment of Cerebral Aneurysms: Comparison between Endovascular Coil Embolization and Surgical Clipping
- Coil Embolization for Distal Middle Cerebral Artery Aneurysm
- Microsurgical Clipping and Coil Removal of Previously Coiled Regrowing Cerebral Aneurysms